


Fluorescence
Many compounds have the nice and interesting property of fluorescence. These compounds capture light (photons) of a certain minimum energy content and emit a photon of a specific frequency with a lower energy. The energy difference is dissipated as heat.
The picture below shows a black light tube, which hardly emits visible light, but the erlenmeyer and the solid start 'glowing' in the dark, due to fluorescence.

![]()
Required equipment:
- Ultraviolet light source (black light or UV LED's).
- Fluorescent dyes or chemicals (fluorescein, optical brightener, paper)
Safety:
- Use an ultraviolet light source, such as a black light (UV around 370 nm) or a UV LED (UV around 390 nm). Do not use a germicidal mercury lamp, with UV light at 254 nm. The latter is very harmful and may cause severe skin burns or cancer.
![]()
Setup of the experiment
This is a very simple experiment. Switch on an ultraviolet light source and keep the objects or compounds with fluorescent properties near the light source. Best results are obtained if the experiment is performed in an almost dark room and the light source emits no or only small amounts of visible light. A standard black light gives good results.
It is nice to see that there are different compounds, which show fluorescence at different wave lengths. A well known and very strongly fluorescent compound is fluorescein, a certain dye. This dye absorbs photons with a wave length shorter than 494 nm, and it emits a photon with a wave length of 520 nm (lime green light). The energy difference is converted to heat in the form of vibration of the molecule.
Soap, used for washing clothes, frequently has a fluorescent dye added as well. This dye absorbs photons with a wave length of less than appr. 400 nm and emits photons with a wave length of appr. 470 nm (blue light). This dye gives an optical illusion of "whiter than white" fabric, especially in sunlight, which contains quite some UV light Many white sheets of paper also contain such a blue fluorescent dye.
Another interesting compound, which shows strong fluorescence is uranyl nitrate. This compound only shows fluorescence in the solid state, its solutions in water do not show fluorescence.
Fluorescence of fluorescein
Fluorescein must be dissolved in an alkaline liquid, such that the anion, instead of the free acid, is formed. This anion has strong fluorescent properties. The compound itself has a yellow color, it absorbs light in the blue part of the spectrum and in the UV-part of the spectrum.
The pictures below show the compound in dim daylight, and in the same light, but with the black light switched on as well. The upper pictures already shows some fluorescence, even without the black light switched on. This is because the blue part of the visible light also causes fluorescence in this compound.


Two videos were made of pouring the non-fluorescent free fluorescein acid into an alkaline solution (2% ammonia), in which the anion of fluorescein is formed. These videos nicely show the formation of the fluorescent compound:
Both files have a download size of approximately 1 MByte.
Fluorescence of optical whitener dye
The following pictures show a very dilute solution of an optical whitener dye, such as used in washing powder for clothes. The solution is colorless in dim daylight, but when a UV light source is switched on, it emits a bright blue light.

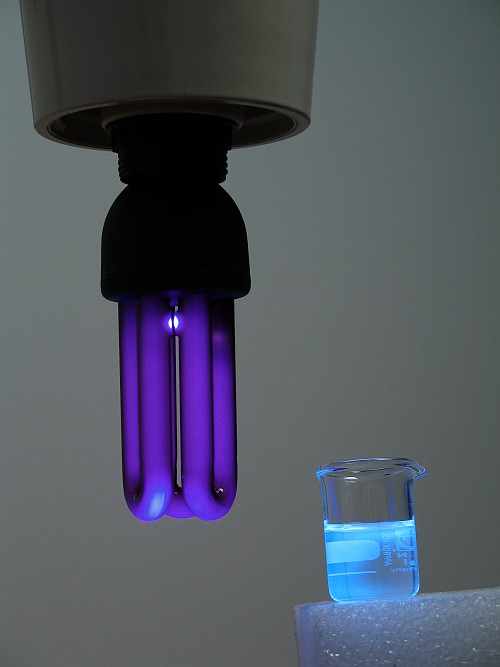
The second picture also nicely shows that the optical brightener dye absorbs UV-light quite strongly. The right side of the little beaker does not show the fluorescent blue light. All UV-light is absorbed in the left part of the beaker and there the fluorescent light appears. The lower-right side of the beaker still shows the colorless liquid. Again, a video is made, now of pouring the blue fluorescent dye in an erlenmeyer, filled with water. Download size is approximately 1.2 MByte.
Fluorescence of white paper
Some sheets of white paper also show fairly strong fluorescence. This is due to the addition of optical brightener dye to the bleached white paper.

This picture nicely shows the difference between the paper and the block of foam, on which the paper is put. In daylight, both of these appear white. But the block of foam does not show any fluorescence, while the paper does.
Yellow fluorescence
Another interesting fluorescent compound is the copper(I) pyridine iodide complex with chemical formula [Cu:NC5H5]I.
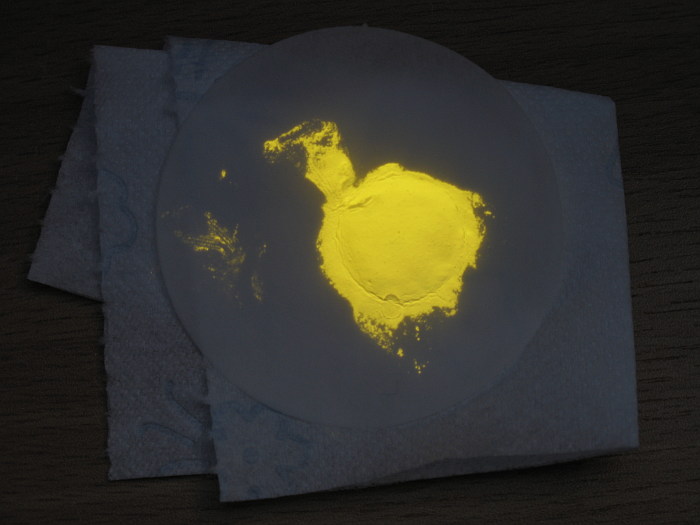
![]()
Discussion of results
When light hits an object, then usually part of the light is absorbed and the rest of the light is reflected or transmitted. Which light is absorbed and which is reflected or transmitted determines the color of objects.
The absorbed energy usually is converted to heat. Each time when a photon hits a molecule, the molecule starts shaking a little bit more. We observe this shaking of the molecules as heat.
Some molecules, however, can go into an excited state when they absorb a photon. In this excited state, the configuration of electrons in the molecule is changed to a higher energy level. If this is the case for a molecule, then only photons with energies exceeding a certain minimum value lead to the molecule going into the excited state. Lower energy photons simply lead to heating up. An excited molecule can remain in that state for a while, but in most cases, it falls back to the normal state within nanoseconds and when doing so, it emits a photon of a very specific wavelength. If this emitted photon is in the visible light range, then we see light, which initially was not present. The very specific wavelength is due to the precise energy difference between the excited quantum state and the normal quantum state of the molecule.
Fluorescein is an example of such a compound. It absorbs photons with wave lengths not exceeding 494 nm and then it goes into an excited state. After a very short time, the molecule falls back to its normal state and the energy released in this process is emitted as a photon of 520 nm and the remaining little amount of energy is converted to heat.
So, in general, fluorescence is a two stage process.
- Absorption of a photon of sufficiently short wavelength. When this occurs, a very specific amount of energy is 'consumed' by going to an excited state and the remaining energy is converted to heat.
- Fallback from excited state to normal state. In this process, again a very specific amount of energy is emitted in the form of a photon of a specific wavelength. Another part of the energy is converted to heat.
If the difference in wave length between absorbed photon and emitted photon is large, then the effect is quite spectacular.