Strong green fluorescence
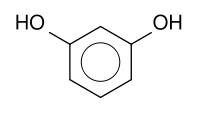
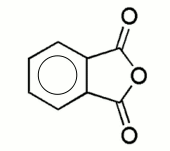
This page contains a description of how fluorescein can be made from two fairly common aromatic chemicals, resorcinol, C6H6O2 and phthalic anhydride, C8H4O3.
 |
 |
|
| resorcinol | phthalic anhydride |
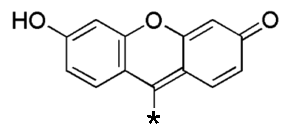
Fluorescein is a brown/red solid, which is only very sparingly soluble in water, and which has a fairly complicated structure, given in the picture at the right. The net formula of fluorescein is C20H12O5.

In alkaline environments, it dissolves more easily. Such a solution is brown/yellow. However, when light of sufficiently short wavelength is falling on this solution, then a remarkable and beautiful green light is emitted by the solution. The picture below shows an alkaline solution of this compound, with a beam of light from a UV-LED passing through the liquid. This produces a bright green light.

![]()
![]() Required
chemicals:
Required
chemicals:
-
phthalic anhydride
-
resorcinol
-
concentrated sulphuric acid
-
sodium hydroxide
![]() Required
equipment:
Required
equipment:
-
test tube
-
burner, for heating the test tube
-
UV-LED plus suitable current limiting resistor and power supply: a 12 V to 15 V voltage source and a series resistor of 500 Ω to 1000 Ω are suitable.
![]() Safety:
Safety:
- Concentrated sulphuric acid is very corrosive. Avoid any contact with the skin, use gloves when handling the concentrated acid.
- Sodium hydroxide also is very corrosive.
![]() Disposal:
Disposal:
- The liquid can be flushed down the drain with a lot of water.
Remark: This experiment also works with succinic anhydride, instead of phthalic anhydride. See at the bottom of this webpage for more info on that.
![]()
Reaction of phthalic anhydride and resorcinol
![]() Take 50 mg of phthalic anhydride and put this in a dry test
tube.
Take 50 mg of phthalic anhydride and put this in a dry test
tube.
![]() Take 75 mg of resorcinol and add this to the phthalic
anhydride. Shake a little, such that the two powders are mixed.
Take 75 mg of resorcinol and add this to the phthalic
anhydride. Shake a little, such that the two powders are mixed.
![]() Add a big drop of concentrated sulphuric acid to the mix of
the two solids and gently heat for a minute or so. This results in
formation of a red liquid, which sticks to the glass and looks yellow in thin
layers.
Add a big drop of concentrated sulphuric acid to the mix of
the two solids and gently heat for a minute or so. This results in
formation of a red liquid, which sticks to the glass and looks yellow in thin
layers.


The left picture shows the mix of the resorcinol and the phthalic anhydride. The resorcinol is off-white (which is common for resorcinol, although really pure resorcinol is purely white). The phthalic anhydride is purely white. The right picture shows the material, after adding the sulphuric acid, and after careful heating.
![]() Let
the red liquid cool down and then at once add some water. This results in
mixing of the red liquid with the water and a yellow solution is formed, with
some small yellow particles in it.
Let
the red liquid cool down and then at once add some water. This results in
mixing of the red liquid with the water and a yellow solution is formed, with
some small yellow particles in it.


The left picture shows the yellow liquid, immediately after adding the water, the right picture shows the liquid a few minutes later, when all small solid particles have settled at the bottom.
![]() Add the
yellow liquid (without the solid particles) to an erlenmeyer, which has
approximately 100 ml of water in it. Plain tap-water may be used for this. Swirl
the liquid in order to obtain a homogeneous solution.
Add the
yellow liquid (without the solid particles) to an erlenmeyer, which has
approximately 100 ml of water in it. Plain tap-water may be used for this. Swirl
the liquid in order to obtain a homogeneous solution.

The solution is yellow, it does not look very special. This liquid is not fluorescent.
![]()
Making the liquid fluorescent by adding excess NaOH
The liquid, shown above, is fairly acidic. The sulphuric acid from the red liquid is dissolved in the water. It can be made alkaline by adding an excess of solid sodium hydroxide and then swirling the erlenmeyer. If this is done, then one clearly sees that at the pearls of sodium hydroxide, the color changes from bright yellow to yellow/brown. Under a white light source, however, the brown/yellow solution clearly shows some fluorescence already.
Nice green light can be observed inside the liquid, but only if white light is shining directly on the liquid. When the liquid is placed behind a wooden bar, such that no white light is shining directly on the erlenmeyer, then one only sees the dull yellow/brown color. Below follow two pictures, made immediately after each other. The first one is without white light, falling on the erlenmeyer, the second one is with white light falling directly on the erlenmeyer.


![]() When the liquid is allowed to stand for a while (all pearls
of sodium hydroxide dissolve) and then swirled, then one can nicely see a
light-emitting vortex in the liquid, which is at the interface between the lower
alkaline liquid (which is more dense) and the acidic liquid above it.
When the liquid is allowed to stand for a while (all pearls
of sodium hydroxide dissolve) and then swirled, then one can nicely see a
light-emitting vortex in the liquid, which is at the interface between the lower
alkaline liquid (which is more dense) and the acidic liquid above it.

![]() Finally, when the liquid is homogeneous again and all of it
has become alkaline, then the fluorescence is visible near the surface. Deeper
inside the liquid, there is no fluorescence. The white light cannot penetrate
deeply into the liquid.
Finally, when the liquid is homogeneous again and all of it
has become alkaline, then the fluorescence is visible near the surface. Deeper
inside the liquid, there is no fluorescence. The white light cannot penetrate
deeply into the liquid.

The two pictures below nicely show the color of transmitted light and the color of the fluorescence. The left picture is made by using the camera flash unit. It shows the shadow of the light, transmitted through the liquid. This light is yellow/brown. The fluorescence is very strong with the flash light. The right picture shows a UV-LED, and the beam of light from this LED is allowed to pass through the liquid. Only where the light is going through the liquid, the strong green fluorescence is observed.


Two videos were made of the fluorescence as well. The erlenmeyer with the liquid moves through the beam of UV-light of the LED and only if this light is passing through the liquid, then the fluorescence can be observed. Otherwise, only the dull brown/yellow color of the liquid can be observed. The videos were made in a dimly lit room.
![]()
Discussion of results
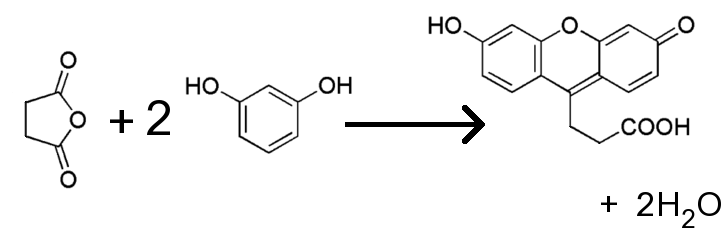
![]() At elevated temperature, and in the presence of a compound, which absorbs water,
phthalic anhydride and resorcinol react with each other, according to the
following net equation.
At elevated temperature, and in the presence of a compound, which absorbs water,
phthalic anhydride and resorcinol react with each other, according to the
following net equation.
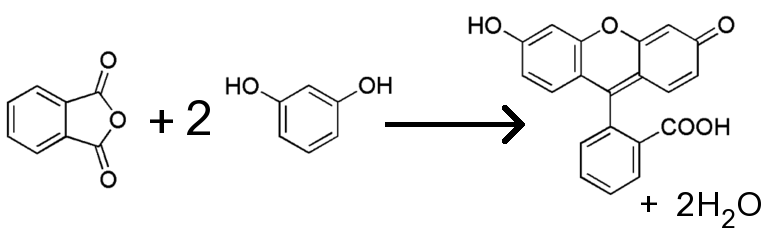
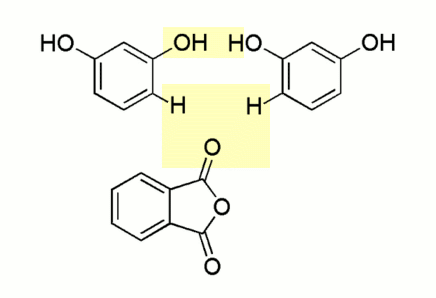
![]() The actual mechanism of the reaction is somewhat more
involved. Phthalic anhydride and two molecules of resorcinol condense into a
single molecule of fluorescein. In the process, two molecules of water are split
off and are absorbed by the sulphuric acid:
The actual mechanism of the reaction is somewhat more
involved. Phthalic anhydride and two molecules of resorcinol condense into a
single molecule of fluorescein. In the process, two molecules of water are split
off and are absorbed by the sulphuric acid:
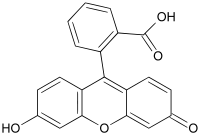
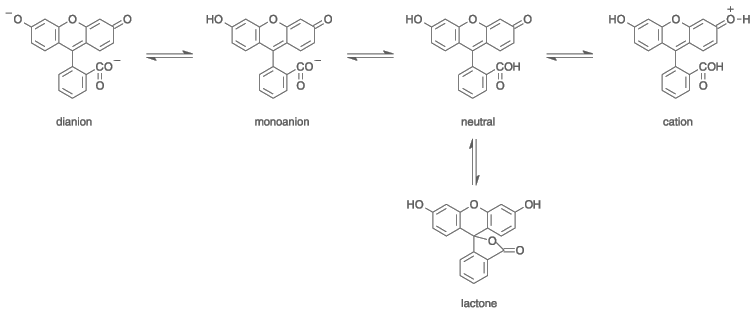
As a result of this condensation, initially fluorescein is created in a slightly different form, and it is this form, which gives rise to the yellow acidic solution:
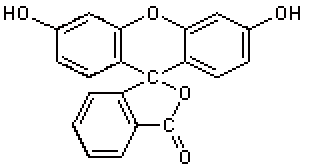
After its formation, two different forms of fluorescein will coexist in an equilibrium. The molecule is rearranged, one H-atom from an -OH group moves to the C(=O)O- at the bottom right and a subsequent shift of electrons occurs in the molecule, leading to the structure, shown above in the net equation, just after the arrow.
For the interested ones, capable of reading German texts, the following document gives a much more in-depth description of the mechanism of formation of the fluorescein:
http://www.mgh.schulnetz.hamm.de/faecher/chemie/farbstoffe/Fluorescein.doc
![]() The fluorescein molecule itself has no fluorescence
properties. The dibasic ion has fluorescence properties. In a solution of NaOH,
two protons can be released, and the following ion is produced.
The fluorescein molecule itself has no fluorescence
properties. The dibasic ion has fluorescence properties. In a solution of NaOH,
two protons can be released, and the following ion is produced.
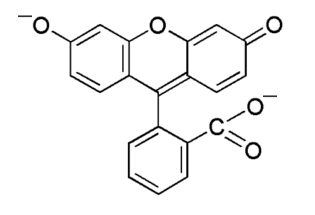
This ion is brought into an excited state by sufficiently short wavelength photons and when it falls back, it emits a photon at approximately 520 nm (green light). For excitation, a photon, hitting the ion must have a wavelength not larger than 494 nm. The difference in energy of the exciting photon and the emitted photon is converted to vibration of the ion and hence it is converted to nothing more than heat.
The UV-LED, used in this experiment, emits a small amount of visible light (blue-violet), but most of the emitted light is ultraviolet. This makes the fluorescence particularly appealing, because the only weakly visible light from the UV-LED causes a very bright fluorescence in the solution. The UV-light also penetrates into the liquid deeply, allowing a nice beam of visible light to be formed in the solution.
![]() A complete overview of all equilibrium reactions, occurring with fluorescein and
the ionized versions is given below. At the left, the situation is given for
high pH (alkaline solution), at the right the situation is given for low pH
(acidic). When the pH goes from high to low, then the equilibrium gradually
moves from left to right. The unionized molecule can exist in two
forms, as already described above.
A complete overview of all equilibrium reactions, occurring with fluorescein and
the ionized versions is given below. At the left, the situation is given for
high pH (alkaline solution), at the right the situation is given for low pH
(acidic). When the pH goes from high to low, then the equilibrium gradually
moves from left to right. The unionized molecule can exist in two
forms, as already described above.
![]()
Replacement of phthalic anhydride with succinic anhydride
In phthalic anhydride there is a structure with 4 C-atoms and a single O-atom, in a 5-membered ring. Such a structure also is present in succinic anhydride, the only difference being the absence of the aromatic ring.
At the left, phthalic anhydride is shown, at the right, succinic anhydride is shown.

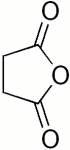
In the experiment, described above, the 5-membered ring is the active part of phthalic anhydride, the aromatic ring does not take part in the reaction. So, the question was, can a fluorescent compound be formed from resorcinol and succinic anhydride? The answer to this question is yes.
The above experiment was repeated, but instead of using approximately 50 mg of phthalic anhydride, approximately 35 mg of succinic anhydride was used. The amount of resorcinol again was 75 mg and a drop of sulphuric acid was added.
The reaction is expected to be as follows (this is not a documented reaction, but it is expected to be correct, due to the analogy with the formation of fluorescein):
The compound, shown at the right was expected to have properties, close to those of fluorescein.
The experimental steps, described above, were repeated with succinic anhydride. The melt of sulphuric acid, resorcinol and succinic anhydride is red/brown. After strong dilution, a yellow liquid is obtained, which does not show any fluorescence. This yellow liquid is very much like the liquid, obtained with phthalic anhydride. Finally, an alkaline liquid was obtained, which indeed shows fluorescence. This final result is shown in the two pictures below.


The liquid, obtained with succinic anhydride, is more orange/brown than the liquid, obtained with phthalic anhydride. The fluorescence is less strong, but still it is remarkable. So, indeed, with succinic anhydride a fluorescent compound is formed as well and the color of the fluorescence is the same (or very close).
Based on the result of the two different experiments, it is assumed that the structure, responsible for the fluorescence, is the group, shown below: