


A fluorescent complex of copper(I)
Sometimes there are such experiments, which are old and forgotten curiosities from the past and this is an example of such an experiment. In this experiment a complex of copper(I) and pyridine is prepared, which shows bright yellow fluorescence when ultraviolet light falls on it.
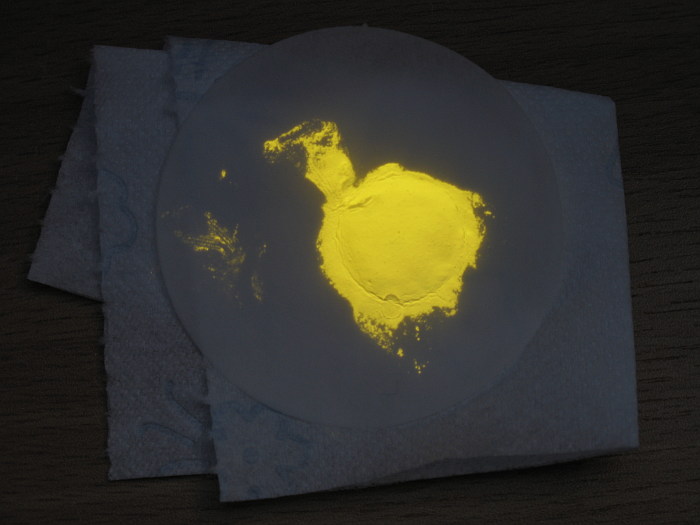
The complex can be prepared very easily and can also be isolated easily, such that a nice sample can be made, which can be stored for display purposes.
Another similar complex can be produced as well, with more pyridine coordinated to copper. This complex is less stable, but shows beautiful cyan fluorescence. Both complexes were ampouled under water for display purposes.

![]()
![]() Required
chemicals:
Required
chemicals:
-
copper(I) iodide, a.k.a. cuprous iodide
When no copper(I) iodide is available, you easily can prepare it from
-
copper sulfate
-
potassium iodide
-
sodium sulfite
-
dilute sulphuric acid
-
-
potassium iodide
-
acetone
-
pyridine
![]() Required
equipment:
Required
equipment:
-
test tubes
-
filter paper
![]() Safety:
Safety:

-
Pyridine is very toxic and has a horrible smell. Avoid exposure to this chemical, both contact with the skin and inhalation of the vapor is very harmful.
-
Copper(I) iodide is harmful and in the dry state easily forms dust. Avoid inhaling of this dust when handling the dry material.
-
Acetone is volatile and highly flammable. At high concentration, the vapor of acetone is somewhat irritating, but acetone is only slightly toxic.
![]() Disposal:
Disposal:
- The liquid and precipitates should be processed as chemical waste and should not be flushed down the drain. Both the pyridine and the copper content are problematic for the environment. Before collecting the waste, one can choose to let most of the acetone in the waste evaporate, before doing it in the waste bottle.
![]()
Preparation of the fluorescent copper(I)/pyridine complex
![]() Take a
small spatula of copper(I) iodide and add two spatulas of potassium iodide. Add
a few ml of water. Precise quantities are not critical. The potassium iodide
quickly dissolves and part of the copper(I) iodide also dissolves. A pale yellow
solution is obtained, with some remaining solid copper(I) iodide on the bottom.
Take a
small spatula of copper(I) iodide and add two spatulas of potassium iodide. Add
a few ml of water. Precise quantities are not critical. The potassium iodide
quickly dissolves and part of the copper(I) iodide also dissolves. A pale yellow
solution is obtained, with some remaining solid copper(I) iodide on the bottom.
This solution and the remaining solid copper(I) iodide does not have any interesting fluorescent properties.
![]() In
another test tube, put 3 ml of acetone and then add 5 drops of pyridine (appr.
0.25 ml). Swirl a little bit in order to dissolve all of the pyridine. It easily
mixes with the acetone. Do this step outside, or in a very well ventilated room.
The vapor of the pyridine really is nasty and should not be inhaled. This 0.25
ml of pyridine is much more than the amount of copper(I) iodide used in this
experiment, so one can safely assume that excess pyridine is used in this
experiment.
In
another test tube, put 3 ml of acetone and then add 5 drops of pyridine (appr.
0.25 ml). Swirl a little bit in order to dissolve all of the pyridine. It easily
mixes with the acetone. Do this step outside, or in a very well ventilated room.
The vapor of the pyridine really is nasty and should not be inhaled. This 0.25
ml of pyridine is much more than the amount of copper(I) iodide used in this
experiment, so one can safely assume that excess pyridine is used in this
experiment.
![]() Add the
acetone/pyridine mix to the test tube in which there is the potassium
iodide/copper(I) iodide solution with
some solid copper(I) iodide still at the bottom. Immediately after adding the
acetone/pyridine mix, it seems as if all solid material dissolves, but almost
immediately a dense flocculent precipitate is formed, which quickly settles at
the bottom. The pale yellow color of the potassium iodide/copper(I) iodide solution even becomes paler.
When all of the precipitate has settled, a white layer remains under an almost
colorless liquid.
Add the
acetone/pyridine mix to the test tube in which there is the potassium
iodide/copper(I) iodide solution with
some solid copper(I) iodide still at the bottom. Immediately after adding the
acetone/pyridine mix, it seems as if all solid material dissolves, but almost
immediately a dense flocculent precipitate is formed, which quickly settles at
the bottom. The pale yellow color of the potassium iodide/copper(I) iodide solution even becomes paler.
When all of the precipitate has settled, a white layer remains under an almost
colorless liquid.
The picture below shows the white material, just after swirling the test tube. The precipitate was just spread through the liquid again.

![]()
Showing the fluorescence of the complex
The picture above shows a black-light tube, which emits near-UV light (around 385 nm) when it is switched on. When the black-light is switched on, then the amount of visible light only slightly increases. The black-light tube seems to give off some weak blue/purple light, but the flocculent solid in the test tube beautifully lights up, showing bright yellow fluorescence.

![]()
Properties of the settled precipitate
The precipitate is flocculent, but despite of that, it settles at the bottom fairly quickly. It only takes a few minutes to have a compact layer at the bottom with a clear liquid above it. The picture below shows the precipitate, settled at the bottom, while sunlight is shining on it. The picture also shows that the liquid above the precipitate is almost colorless, it just has a faint yellow color. In the sunlight the precipitate looks white, no fluorescence can be observed.

When the window is blinded, such that no direct sunlight comes into the room and the test tube is kept near the black-light, then the result is as follows:

![]()
Isolation of the complex
Getting the material in a reasonably pure state is not very difficult. Let the material settle at the bottom as in the pictures shown above and then carefully pipette away the layer of liquid above the precipitate. Then add a lot of distilled water and shake the test tube, such that all of the precipitate is mixed up with the fresh water. Then let it settle at the bottom again and then carefully pipette away the water again. This procedure then can be repeated another time.
The rinsed precipitate next can be put on a piece of filter paper, which is put on a piece of household tissue or toilet tissue. By carefully tapping the test tube with the settled precipitate in it, it slowly can be tapped out onto the piece of filter paper. All water mixed with the precipitate now is absorbed by the tissue and the material remains on the filter paper in an almost dry state.
Further cleaning can be done by putting the material on a fresh dry paper tissue and carefully pouring another 2 ml of distilled water on the solid material. After this treatment, the solid material has no smell of pyridine anymore and it can be regarded quite pure after all those rinses with water.
The pictures below show the material on the filter paper in a dimly lit room and in the same place, but now with the black-light switched on. The filter paper and tissue also look slightly lighter in the light of the black-light, but the yellow fluorescence is really strong compared to the reflected light of the paper.

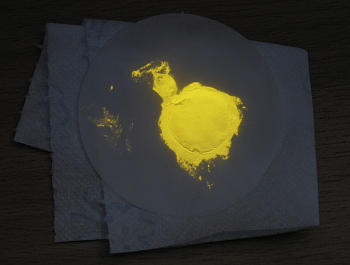
If a good quality filter paper is used, then the material easily can be scraped off after drying. Do not tap the material directly on the toilet tissue if you want to scrape it off. Toilet tissue or household tissue has much wider pores than the filter paper and the material hardly can be scraped off after drying without also tearing off a lot of paper.
Making and isolating a larger quantity of the pyridine-complex can be done as follows:- Put 5 grams of finely powdered or finely suspended copper(I) iodide in a beaker.
- Put 7.5 grams of potassium iodide in the same beaker.
- Add 25 ml of water and swirl, until all copper(I) iodide is suspended in the liquid. Part of it will dissolve, but not all of it. You get a yellow solution over a tan/off white solid.
- In a separate small beaker, pour 4 to 5 ml of pyridine.
- Add appr. 20 ml of acetone to the pyridine and mix the liquids.
- Slowly, while stirring, add the 25 ml of acetone/pyridine mix to the suspension of copper(I) iodide/potassium iodide.
- The yellow color fades, a pure white flocculent solid is obtained, which fairly quickly settles at the bottom. Stir occasionally, allow to react for 5 minutes.
- Add a lot of water, while stirring.
- Allow white solid to settle, decant off (or pipette away) as much as possible of the water without taking white solid with the decanted water.
- Add a lot of water again, while stirring and decant again. Repeat two times to get rid of most impurities.
- Filter the remaining liquid with the white solid in it. Keep the white solid and press dry between filter paper, with a lot of paper tissue around the filter paper. Assure that the material does not come in direct contact with the paper tissue.
- The now just damp material can be put on a petri dish. Allow to dry for several hours at a warm place, free of dust. Do not heat!
- The resulting material has a pale tan/off white color, is perfectly dry and shows strong fluorescence under UV-light.

The total amount, shown here is just over 6.5 grams.
Half a gram of the dry powder is shown in three pictures below, with light from a TL-bar, with combined TL-light and UV-light, and with UV only. Lighting settings on the camera is the same in all three pictures.



![]()
Another complex with twice as much pyridine per copper atom
It is possible to make another complex with copper(I) iodide and pyridine. This complex, however, is less stable and only can be kept around, suspended in water with an excess amount of pyridine dissolved in it. This complex has a pale yellow color and it shows beautiful green/cyan fluorescence when UV-light falls on it.
Making this complex can be done as follows:
- Put 0.5 grams of finely powdered CuI in a test tube.
- Add 0.6 grams of KI to the same test tube.
- Add a few ml of water and swirl. Part of the CuI dissolves, most of it remains suspended in the liquid.
- In a separate test tube put 1 ml of pyridine.
- Add a few ml of water to the pyridine and swirl, so that the pyridine and water mix.
- Add the pyridine/water mix to the CuI/KI suspension and shake. Allow to react, while occasionally shaking, for a few minutes.
- A pale yellow precipitate is formed. Add a lot of water and swirl. Allow to settle and decant most of the liquid. Repeat this procecure a few times to get rid of most impurities.
- Finally, add a mix of 20 ml of water and 0.5 ml of pyridine to the pale yellow solid and swirl. Keep it in this way, suspended in the liquid.
In this way, if your
rinse/decant process does not have too many losses, you will have appr.
0.9 grams of the pyridine complex suspended under water.
If the product is kept under pure water, or if one tries to dry it, then it loses pyridine and becomes non-fluorescent, or under some conditions, it turns into the complex with yellow fluorescence.
Both complexes were
ampouled. This was done by preparing a small amount of complex and then
rinsing with a lot of water to get rid of impurities. The complex with
yellow fluorescence is pure white, the complex with green/cyan
fluorescence is pale yellow. The white complex is ampouled under plain
water, the yellow complex is ampouled under a 5..10 % solution of
pyridine in water. If no excess pyridine is present, then the yellow
complex slowly decomposes and loses its fluorescent properties. The
picture below shows both ampoules.

The three pictures below show both ampoules under TL-light, a combination of TL-light and UV-light and UV-light only.



![]()
Procedure for making copper(I) iodide.
- Dissolve 2 grams of anhydrous sodium sulfite in 20 ml of water and add 10 ml of 15% sulphuric acid. This results in a fairly strong smell of sulphur dioxide.
- Dissolve 5 grams of copper sulfate pentahydrate in some water (you will need a few tens of ml for this) and add this to the solution of sodium sulfite.
- Dissolve 3.5 grams of potassium iodide in 10 ml of water and add this solution to the other solution. This results in formation of a white (or somewhat off-white) precipitate. The liquid above the precipitate will be colorless or pale yellow.
- Stir the liquid, such that all chemicals react and a lot of precipitate is formed.
- Filter the liquid and keep the solid material. Rinse with distilled water while the solid is in the filter.
- Keep the solid material, stored under water. There is no need to dry the material.
- You can dry the material, but the color of the solid then slightly darkens to a grey or tan color. The material is oxidized somewhat by air while it is drying. If the only goal is to make the pyridine complex, then it is better not to dry the copper(I) iodide, just rinse it very well, such that no acid and sulphur dioxide remains attached to it, and use the compound immediately after its preparation.
![]()
Discussion of results
![]() When copper(I) iodide is added to a solution of excess potassium iodide, then a
soluble copper complex is formed:
When copper(I) iodide is added to a solution of excess potassium iodide, then a
soluble copper complex is formed:
CuI + I– ↔ CuI2–
This copper(I) complex easily breaks down again, hence the ↔ in the equation. Dilution causes reprecipitation of CuI. Taking away CuI or taking away free iodide ion also forces the reaction to proceed completely to the left.
![]() When pyridine is added, then an insoluble complex is formed:
When pyridine is added, then an insoluble complex is formed:
4 CuI2– + 4 :NC5H5 → Cu4(:NC5H5)4I4 + 4 I–
The nitrogen atom in the pyridine has a free electron pair, denoted by a : symbol. This free electron pair is coordinated to a copper atom. One iodide ion from the CuI2– complex splits off and goes into solution, the other iodide remains coordinated to the copper atom and becomes coordinated to other copper atoms as well in the complex.
The release of free iodide ion leads to increase of
concentration of iodide and any solid CuI at the bottom of the test tube then
dissolves as the diiodocopper(I) complex which in turn also reacts with
pyridine. All available copper(I) finally is trapped in the highly insoluble
pyridine complex. The complex Cu4(:NC5H5)4I4
shows yellow fluorescence at room temperature. This complex has a
rather complicated structure, with at its core a tetrahedron,
consisting of 4 copper atoms.
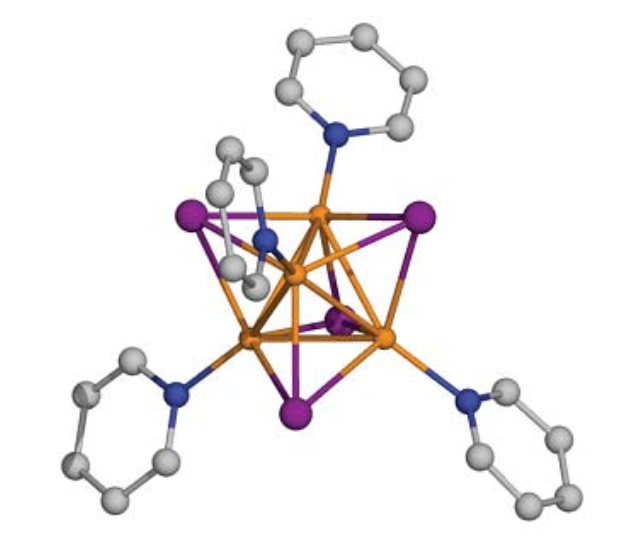
The structure of the complex which exhibits green/cyan fluorescence is not known to me. Its empirical formula is Cu(:NC5H5)2I2.