


Electrolysis of acetate and formiate
This experiment is not really intended as a demonstration of spectacular chemical properties, but it is intended as a demonstration of how some fairly simple theory can be used to derive nice results and to confirm the reasoning with experimental outcomes.
Before the experiment was conducted, the outcome of this experiment was not known beforehand to me as the author of this page. With some reasoning, however, I could establish both the results for acetate and for formiate.
This experiment in fact is a set of two experiments. Experiment 1 is electrolysis of a solution of sodium acetate, with some acetic acid in solution as well. Experiment 2 is electrolysis of sodium formiate, with some formic acid in solution as well. The goal of the experiments is to determine what the net chemical reaction is with the above mentioned chemicals.
The experiments were conducted, using a special apparatus for making the gases, constructed from a U-tube with some aquarium-tubing and rubber stoppers.

![]()
![]() Required
chemicals:
Required
chemicals:
-
formic acid
-
acetic acid
-
sodium hydroxide
![]() Required
equipment:
Required
equipment:
-
test tubes
-
U-tube with side arms
-
rubber tubing, as thin as possible
-
big beaker, tub or something else, in which inverted test tubes can be put
-
power supply (and current limiting power resistor)
-
small syringe
![]() Safety:
Safety:
- Concentrated formic acid and acetic acid are corrosive and give off very pungent vapor.
- Sodium hydroxide is very corrosive and a lot of heat is produced if this is mixed with the acids.
![]() Disposal:
Disposal:
- After use, the solutions can be flushed down the drain.
![]()
Constructing the apparatus for this set of experiments
For this, one is referred to the following web page: detonating gas. This web page has a section dealing with the construction of the electrolysis apparatus for detonating gas. Exactly the same apparatus can be used for the experiments, described in this web page.
![]()
Preparation of electrolytes
In both experiments, a solution of the sodium salt of the acid is used, but the solution is prepared such, that it still is somewhat acidic (tested simply by smelling it, as long as there is pungent smell, it is acidic).
The solutions can be prepared by mixing 2 to 3 ml of concentrated acid (80% acetic acid can be used for the acetate experiment, 85% formic acid can be used for the formiate experiment) with 10 ml of water. To this mix, carefully solid sodium hydroxide must be added in small lots and then it must be dissolved by stirring. One should continue adding, until only a faint acid smell remains. If too much sodium hydroxide is added, then again some acid must be added. Finally, water is added, such that the volume becomes 15 ml.
The reason of keeping some excess acid in solution is that no hydroxide ions must be present in the solution. Acetate and formiate are derived from weak acids, and pure solutions of these have a fairly high pH. Such a high pH results in formation of a fairly high concentration of hydroxide ions in the solution, and that would have a negative effect on the experimental outcome. Hydroxide ions easily lead to production of oxygen at the anode, or even of small amounts of hydrogen peroxide. This side reaction is unwanted, it will spoil volume measurements and tests for the properties of the collected gases. The undissociated acid is not expected to have a negative influence on the precision of the outcome of the experiments.
![]()
What can be expected from electrolysis of acetic acid/acetate?
The acetate/acetic acid solution contains the following species at appreciable concentration:
- H2O
- CH3COO–
- Na+
- CH3COOH
- H+ (concentration of this will be noticeable, but not high, the acid is only weak)
At the cathode, things are simple. One can expect hydrogen gas to be formed, either by reduction of water, or by reduction of hydrated H+ ions. If any hydroxide is formed, then it immediately is neutralized by excess CH3COOH.
At the anode, things are more complicated. It is known that acetate ion is not easily oxidized. Given the other species in solution, one could expect oxygen to be formed. However, acetate ion is attracted by the anode with positive potential. If it is discharged, then a species CH3COO· is formed, which is not stable. Such a species could attract hydrogen from water, it also could decompose, giving CO2 and ·CH3. Maybe different compounds are produced at the same time, such as oxygen, carbon dioxide and organic compounds. CO2 would escape as gas, the radical ·CH3 could react with many species, two such radicals could combine to ethane, H3CCH3. If oxygen is produced, it also escapes as gas.
![]()
Experimental results with acetic acid/sodium acetate as electrolyte
The observed current is approximately 0.25 A.
The ratio of volume of gases from cathode and anode is somewhat erratic. Multiple experiments show that the ratio is somewhere around Vcathode : Vanode ≈ 1 : 2.5, but there is quite a lot of variation. In this particular experiment, three runs resulted in a ratio Vcathode : Vanode being approximately
- 1 : 2.7
- 1 : 2.1
- 1 : 2.5
The large variations can be explained by assuming that one (or both) of the gases is fairly soluble in water or that there is some flaw in the apparatus (leaking away of gas).
A test of the cathode gas confirms that it is hydrogen gas. When it is mixed with air, and that mix is ignited, then a whistling/whoosh sound is produced, which is funny on its own. A video with sound can be downloaded (file size is appr. 2.2 MByte).
The anode gas was tested in different ways.
- Does it contain CO2? This is tested by keeping the test tube with the anode gas in a water tub and pressing a concentrated solution of sodium hydroxide along the glass walls from a syringe and then immediately loosely capping it and shaking it (under water, assuring that no bubbles of air but only additional water is sucked in). Any CO2 will be absorbed by the solution of sodium hydroxide.
- Is it flammable? This is tested by trying to ignite some of the anode gas. A similar test is done after the CO2-test with remaining gas (if something remains).
The first test reveals a very specific property, which only became apparent, when it was done multiple times. The volume of anode gas is somewhat erratic, but the volume of gas, remaining after absorption of carbon dioxide always is exactly the same as the volume of gas, collected at the cathode! The following pictures show this. The left picture shows the collected gases, from one experiment. Left is the anode gas, right is the cathode gas. The volume of anode gas in this particular experiment is roughly 2.5 times the volume of cathode gas. The right picture shows the volume of remaining anode gas, after part of it is absorbed by a sodium hydroxide solution. The remaining volume of anode gas is precisely the remaining volume of cathode gas (there are some bubbles, due to the shaking and the presence of the sodium hydroxide, but the volumes are very close).


So, this part of the experiment shows that the anode gas is a mix of at least two gases. One of the gases is absorbed by a solution of sodium hydroxide. Given the formula of acetate ion, this only can be carbon dioxide. The other gas (or mix) is produced in a precise 1 : 1 ratio with hydrogen gas from the cathode.
The fact that the remaining anode gas always occupies exactly the same volume as the collected cathode gas rules out a flaw in the apparatus. When there was a flaw, resulting in leaking away of gas, then this precise 1 : 1 ratio would not be observed for all experiments.
The gas from the left tube was sucked into a syringe. The picture below shows a syringe, filled with approximately 6 ml of gas, and a little piece of tubing and a glass dropper attached to it. Also a few drops of water are sucked into the syringe, but this is not a real concern.
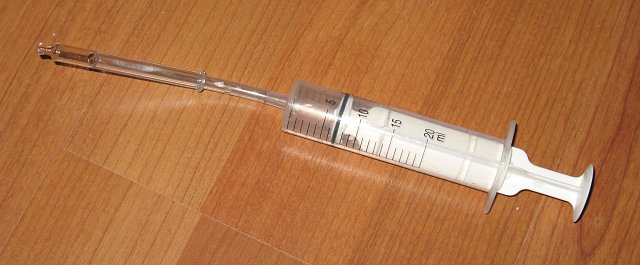
The gas from this syringe can slowly be pressed into the air and this gas can be ignited and keeps burning, as long as there is a slow flow of gas. The picture below shows this burning gas. A video is available as well (download size is approximately 2 Mbyte).

Summarizing all observations
Electrolysing sodium acetate/acetic acid gives the following experimental results:
- Gas is produced at the cathode. This is confirmed to be hydrogen gas.
- Gas is produced at the anode, this is a gas mix. This mix contains CO2 and some gas X.
- Volume ratios are VH2 : VX = 1 : 1 and VH2 : VCO2 ≈ 1 : 1.5, but this latter ratio is somewhat erratic.
- X is flammable.
Explanation of observations
At the cathode there is the following reaction:
2H2O + 2e → 2OH– + H2
At the anode, it is assumed that the carbon dioxide is from the acetate ion. For each two electrons at the cathode, two electrons are absorbed at the anode. One can assume the following:
2CH3COO– – 2e → 2CH3COO·
The experiment shows that carbon dioxide is formed. Based on that observation, it is assumed that the radicals decompose:
2CH3COO· → 2CH3· + 2CO2
Based on this, one would expect VH2 : VCO2 = 1 : 2. The observed value always is lower and shows large variation around the ratio 1 : 1.5. This, however, can be explained. Carbon dioxide dissolves in water (especially cold water) quite well. The variation can be explained, because the bubbling process is variable. Sometimes there were few big bubbles, other times, there were more smaller bubbles. In one of the experiments, some time passed between stopping the collection of bubbles and capping the test tube, allowing more gas to dissolve. Probably other reasons can be imagined as well.
What remains to be explained is one volume of gas X. For each molecule of H2 there must be precisely 1 molecule of X. As the COO part of the acetate already is used for the carbon dioxide, only two CH3· radicals remain. Two of these, combined to one molecule of ethane, H3CCH3, perfectly explain the properties of gas X (flammability) and the ratio of volumes.
So, at the anode, the net reaction most likely is
2CH3COO– – 2e → H3CCH3 + 2CO2
Total net reaction of electrolysis of acetate/acetic acid
Combining the cathode reaction and anode reaction one gets the following:
Reduction of water at cathode: 2H2O + 2e → 2OH– + H2
Immediate neutralization of hydroxide: 2OH– + 2CH3COOH → 2H2O + 2CH3COO–
Net reaction at anode: 2CH3COO– – 2e → H3CCH3 + 2CO2
When all these equations are added, and molecules and ions, which are at the left and at the right are removed from the equation, then the following grand net equation remains:
2CH3COOH → H2 + H3CCH3 + 2CO2
![]()
What can be expected from electrolysis of formic acid/formiate?
Now, with the results of the acetate electrolysis available, one can think about what could happen with formiate. If a completely analogous reaction is occurring, then one would expect for each volume of hydrogen at the cathode, one volume of hydrogen at the anode and two volumes of carbon dioxide. This can be explained by replacing the CH3· radical of the acetate ion by a H· radical from the formiate ion in all anode reactions.
In an experiment, very similar to the experiment with acetate/acetic acid, it was tested whether this analogy holds or not.
Experimental results with formic acid/sodium formiate as electrolyte
The experimental outcome of the formic acid/sodium formiate electrolysis differs a lot from the experimental outcome with acetic acid/sodium acetate. With formiate, the volume of gas, produced at the anode always is less than the volume of gas, produced at the cathode. The ratio again is somewhat erratic. It is varying around Vcathode : Vanode ≈ 1 : 0.7. Multiple experiments give results varying between 1 : 0.6 and 1 : 0.8.
Based on this observation, it already can be deduced that the analogy with acetate electrolysis does not hold here. Based on these new observations, one could expect carbon dioxide at the anode, possibly mixed with oxygen.
Again, the absorption test with sodium hydroxide was done. The result of this is quite surprising. Almost all of the anode gas is absorbed, only a small bubble of gas remains. This final bubble of gas is not analyzed further. The two pictures below show the collected gases before absorption and after absorption.


In each of the pictures, the left test tube is with cathode gas (hydrogen), the right test tube contains the anode gas. In the right picture, only a small bubble at the top of the test tube remains. The only possible explanation is that at the anode only a single gas is produced, being carbon dioxide. The small bubble probably is due to a minor side reaction in which oxygen is produced. It may also be due to some air, which escaped from the water, in which it was dissolved. More analysis would be required to determine what this is. But one thing is for sure, almost 100% of the anode gas is carbon dioxide.
Explanation of observations
At the cathode again there is the following reaction:
2H2O + 2e → 2OH– + H2
At the anode, only carbon dioxide gas is produced. The volume of this gas is less than the volume of hydrogen from the cathode.
If an anode reaction must be written, then initially one is tempted to write an equation, very similar to the one for acetate ion, being the discharge of the formiate ion, resulting in a radical, which is very unstable:
2HCOO– – 2e → 2HCOO·
However, the two HCOO· radicals do not produce 2CO2 as in the case of two CH3COO· radicals, but they apparently recombine to give just a single molecule of CO2. A possible (and most likely) reaction is the following:
2HCOO· → CO2 + HCOOH
This reaction best matches the observations. It tells that cathode gas and anode gas are produced in a 1 : 1 volume ratio. The observation, that less anode gas is collected can be explained again by assuming that part of the CO2 dissolves in the cold water.
Total net reaction of electrolysis of formiate/formic acid
Combining the cathode reaction and anode reaction one gets the following:
Reduction of water at cathode: 2H2O + 2e → 2OH– + H2
Immediate neutralization of hydroxide: 2OH– + 2HCOOH → 2H2O + 2HCOO–
Net reaction at anode: 2HCOO– – 2e → HCOOH + CO2
When all these equations are added, and molecules and ions, which are at the left and at the right are removed from the equation, then the following grand net equation remains:
HCOOH → H2 + CO2
![]()
Chloroacetate instead of acetate, totally different reaction!
After the experiment with acetate/acetic acid, an attempt was made to use monochloroacetic acid instead of acetic acid. Two grams of this solid were dissolved in 10 ml of cold water, and some sodium hydroxide was dissolved as well, but the amount was adjusted, such that pH still remains on the acidic side, somewhere between 3 and 4, tested with some pH paper. When both solids are dissolved, then water is added, such that 15 ml of liquid is obtained and the liquid is allowed to cool down, before it is poured in the U-tube.
So, the electrolyte now contains CH2ClCOOH, CH2ClCOO– and Na+.
If a reaction would occur, which is similar to the reaction of acetic acid, then one would expect the following compounds in the following molar ratio:
VH2 : VCH2ClCH2Cl : VCO2 = 1 : 1 : 2.
As 1,2-dichloroethane is a liquid at room temperature, only carbon dioxide would escape at the anode and droplets of insoluble 1,2-dichloroethane would collect at the bottom of the U-tube.
Experimental results with monochloroacetate/monochloroacetic acid
The actual experiment, using the same power supply and series resistor, however, gives totally different results. The current, going through the cell, starts with less than 0.2 A and after two hours, it has risen to well above 0.3 A. The liquid also has become warm (not hot) after such a time of electrolysis.
Initially, immediately after switching on the power supply, gas production at the anode is faster than gas production at the cathode. The volume ratio of the collected gases is Vcathode : Vanode ≈ 1 : 1.5. When bubbles are counted, then the anode gas even appears somewhat faster and then a ratio of 1 : 2 is approached. The difference most likely is due to dissolving of carbon dioxide in the water, above which the gases are collected. The bubbles of anode gas are odorless.
After two hours of electrolysis, the situation has changed a lot. The volume ratio of the produced gases is Vcathode : Vanode ≈ 1 : 0.7. Bubbles of anode gas have a clearly noticeable smell of elementary chlorine now, although not so strong that it becomes pungent. When the U-tube is opened up after three hours of electrolysis, then the smell of chlorine is very strong and pungent. The liquid around the anode has a clearly visible green/yellow color.
When current is measured, and one is computing how much hydrogen gas is produced, then one also sees a remarkable effect. Initially, much less hydrogen (maybe even 50% less) is produced, than one would expect, based on the current, flowing through the cell. This means that at the cathode, not only hydrogen is formed from water, but there is another reaction as well. After two hours of electrolysis, the volume of produced hydrogen gas corresponds quite well with the current flowing through the cell, at least 90% of all electrons delivered at the cathode are used for formation of hydrogen.
Analysis of the anode gas reveals that 70% to 80% of the anode gas is CO2. This is shown by absorbing the gas into a solution of NaOH, just as with the two experiments above. The remaining gas (or mix of gases) is flammable. When the remaining anode gas is mixed with air in a 1 : 1 volume ratio, and then it is ignited, one sees a strange soft pink flame, with a blue hue.
The 6 pictures below show this effect with tungsten light being used as external light source (hence the yellow background light).






When this effect is watched in daylight, then the flame looks somewhat different:

Now, a blue color is observed, with an orange flame above it.
When ammonia is used for absorbing the carbon dioxide, and the remaining gas is mixed with air and ignited, then a similar flame color is obtained, but without the orange flame above the blue flame.

It might be that the orange color is due to sodium ions, which are dispersed in very tiny droplets in the gas-mix above the liquid. If this indeed is the case, then these sodium ions are from the NaOH, used to absorb the carbon dioxide.
The pure blue color of the flame and the lack of bright flames, due to black body radiation, made me think of carbon monoxide as being one of the main constituents of the flammable gas mix after removal of carbon dioxide. Treatment of the remaining gas mix with a solution of CuCl in dilute HCl indeed shows that approximately 50% of the flammable gas mix is carbon monoxide. Such a copper(I) solution fairly quickly absorbs half of the gas mix when it is shaken with the gas. After removal of the carbon monoxide, still some gas remains. This gas mix in turn was treated with a solution of pyrocatechol in sodium hydroxide. Again, part of the gas mix was absorbed. This means that the gas mix also contains some oxygen. What still remains was tested with a flame. It did not burn. Summarizing the composition of the anode gas:
- carbon dioxide (70% to 80%)
- carbon monoxide (10% to 15%)
- oxygen (5% to 10%)
- remains (roughly 5%)
The remains may be due to air, which still was trapped in the device (during electrolysis air becomes more and more dilute, but still some may be left after two hours of electrolysis). It might also be that the remains are a flammable gas, but it might be that the test for flammability failed, because of the small volume of this gas. The composition of the anode gas also seems to be somewhat variable, hence the very rough estimates of the amounts of the constituents of the anode gas.
Explanation of observations
Explaining the observations is not easy at all. A lengthy discussion on this was started on www.sciencemadness.org (thanks go to user 'len1', who has contributed greatly to my understanding of this reaction and also to my general understanding of this type of reactions). As a final explanation, the following is given:
At the start, at the cathode the following net reaction occurs (highly simplified, the real reaction may be through reduction of water):
2H+ + 2e → H2
Besides the reaction, in which hydrogen gas is produced, another side reaction occurs:
CH2ClCOOH + H+ + 2e → CH3COOH + Cl–
The latter reaction does not result in formation of gas. This explains why initially there is less hydrogen gas, than one would expect on the basis of the current through the cell.
At the anode, things are even more complicated. Initially, as with the acetate reaction, the chloroacetate ion is discharged and a radical is formed:
CH2ClCOO– – e → CH2ClCOO· → CH2Cl· + CO2
The radical CH2ClCOO· falls apart to carbon dioxide, and CH2Cl· appears as a new radical. At this point there is a large difference with acetate ion. The CH2Cl· radicals do not combine to 1,2-dichloroethane, but they are oxidized further. HCl splits off and the radicals are oxidized to CO and CO2.
Equations, which can be written for this are the following:
CH2Cl· + H2O – 3e → CO + 4H+ + Cl–
CH2Cl· + 2H2O – 5e → CO2 + 6H+ + Cl–
These equations result in the following net anode reactions:
(reaction A) CH2ClCOO– + H2O – 4e → CO + CO2 + 4H+ + Cl–
(reaction B) CH2ClCOO– + 2H2O – 6e → 2CO2 + 6H+ + Cl–
Another oxidation could be formation of formic acid. This is very similar to the oxidation to CO. One could see the oxidation to CO also as oxidation to formic acid, followed by decomposition of the formic acid.
CH2Cl· + 2H2O – 3e → HCOOH + 4H+ + Cl–
The last equation does not result in formation of gas at the anode.
If only reaction A occurs at the anode, then for each pair of gas molecules CO+CO2 at the anode, 4 electrons are needed. At a 100% hydrogen production efficiency at the cathode, this means that 2 molecules of gas are produced at the cathode for 2 molecules at the anode. This would result in Vcathode : Vanode = 2 : 2 = 1 : 1.
If only reaction B occurs at the anode, then for each two molecules of CO2 gas at the anode, 6 electrons are needed. At a 100% hydrogen production efficiency at the cathode, this means that 3 molecules of gas are produced at the cathode for 2 molecules at the anode. This would result in Vcathode : Vanode = 3 : 2.
If both reactions A and B occur simultaneously, 50% each of them, then a mix of 75% CO2 and 25% CO is produced, and then Vcathode : Vanode = 2½ : 2.
The latter result fairly well explains the observations. The ratio 2½ : 2 is somewhat high, the true volume ratio is approximately 1 : 0.7. This can be explained by assuming that a little bit less CO is produced, e.g. 40% of the anode reaction is reaction A and 60% is reaction B. In that case, 20% of the produced gas is CO, and the ratio Vcathode : Vanode is closer to 0.7. Dissolved carbon dioxide would bring the ratio somewhat lower, but less than 100% hydrogen production at the cathode compensates for that.
There also is some production of oxygen at the anode, and a tiny amount of chlorine, but this hardly affects the explanation, the experimental errors are larger than the margins in which these gases are produced.