


Preparation of different forms of neodymium sulfate
In this webpage, two different forms of neodymium sulfate are prepared from the same solution. The appearance of the compounds is very different, but when they are dissolved in water, then they look very similar.

Both vials contain neodymium sulfate. The crystals at the left are made by evaporation of a solution of neodymium oxide in dilute sulphuric acid, while the solid at the right is made by boiling a solution of neodymium oxide in dilute sulphuric acid.
![]()
![]() Required
chemicals:
Required
chemicals:
-
neodymium oxide of good purity (99.9% or better)
-
dilute sulphuric acid, 15% by weight
![]() Required
equipment:
Required
equipment:
-
test tubes
-
beaker
-
large watch glass or petri dish
-
means of heating a test tube carefully, e.g. an alcohol burner
![]() Safety:
Safety:
- Dilute sulphuric acid is corrosive, when it comes in contact with the skin, then rinse away with water.
- When dilute sulphuric acid is made from concentrated acid, then slowly add acid to the water, not the other way around and stir well while adding the acid.
![]() Disposal:
Disposal:
- Only small amounts of neodymium are used and this metal only is slightly toxic. The waste can be flushed down the drain.
![]()
Procedure for making neodymium sulfate of high purity
Neodymium sulfate is made by dissolving neodymium oxide in dilute sulphuric acid and then heating the solution. The precise procedure is given below.
![]() Put 40 ml of approximately 15% sulphuric acid in a 100 ml
beaker (you can make this by taking 30 ml of water, adding 3 ml of concentrated
sulphuric acid and then adding more water until you have 40 ml of liquid). Take
5.0 grams of powdered Nd2O3 and add this to the acid in
small portions. Do not add all of the oxide at once.
Put 40 ml of approximately 15% sulphuric acid in a 100 ml
beaker (you can make this by taking 30 ml of water, adding 3 ml of concentrated
sulphuric acid and then adding more water until you have 40 ml of liquid). Take
5.0 grams of powdered Nd2O3 and add this to the acid in
small portions. Do not add all of the oxide at once.
![]() Carefully stir and heat the liquid, until all of the oxide
has dissolved. This takes a fairly long time. When all of the oxide has
dissolved, the liquid has a grey and somewhat yellowish color under TL-light and
a brown/pink color in daylight.
Carefully stir and heat the liquid, until all of the oxide
has dissolved. This takes a fairly long time. When all of the oxide has
dissolved, the liquid has a grey and somewhat yellowish color under TL-light and
a brown/pink color in daylight.
![]() When all
of the oxide has dissolved, then continue heating more strongly until the liquid
starts boiling. When this is done, then quite suddenly a lot of compact
crystalline pink precipitate is formed, which settles at the bottom. If this
happens, then continue heating/boiling, but vigorously stir the liquid while
doing so, otherwise you will have severe (dangerous!!) bumping. Keep on boiling
for one minute or so and then stop.
When all
of the oxide has dissolved, then continue heating more strongly until the liquid
starts boiling. When this is done, then quite suddenly a lot of compact
crystalline pink precipitate is formed, which settles at the bottom. If this
happens, then continue heating/boiling, but vigorously stir the liquid while
doing so, otherwise you will have severe (dangerous!!) bumping. Keep on boiling
for one minute or so and then stop.
![]() Let the
precipitate settle. This only takes a few seconds, the precipitate is very
compact. When it has settled at the bottom, quickly decant the still very hot
liquid above the pink precipitate into another beaker and set this aside (do not
discard this liquid, you need it later on). Decanting is easy, because the
precipitate is compact. Try to decant as much as possible of the liquid from the
precipitate.
Let the
precipitate settle. This only takes a few seconds, the precipitate is very
compact. When it has settled at the bottom, quickly decant the still very hot
liquid above the pink precipitate into another beaker and set this aside (do not
discard this liquid, you need it later on). Decanting is easy, because the
precipitate is compact. Try to decant as much as possible of the liquid from the
precipitate.
![]() Rinse
the precipitate with 20 ml of boiling hot distilled water. The rinse
water becomes yellow as well, but after a few swirls it becomes more like grey
(most likely some of the crystalline material dissolves). Decant the rinse
water. You may discard this liquid. Rinse a second time again with boiling hot
distilled water. When this is swirled, then the rinse water becomes pale
lavender, no yellow color is present anymore. Decant as much as possible of the
second batch of rinse water, such that a compact pink solid mass remains.
Rinse
the precipitate with 20 ml of boiling hot distilled water. The rinse
water becomes yellow as well, but after a few swirls it becomes more like grey
(most likely some of the crystalline material dissolves). Decant the rinse
water. You may discard this liquid. Rinse a second time again with boiling hot
distilled water. When this is swirled, then the rinse water becomes pale
lavender, no yellow color is present anymore. Decant as much as possible of the
second batch of rinse water, such that a compact pink solid mass remains.
![]() Transfer
the still wet precipitate to a piece of filter paper, which is put on a piece of
paper tissue. By doing this, most of the remaining rinse water is absorbed into
the paper tissue, allowing an even purer product. Do not put the precipitate
directly on paper tissue. Filter paper is much stronger and you can easily
scrape off the solid later on, but paper tissue becomes very weak when wet and
you will have a hard time recovering the precipitate when it is on weak and torn
apart paper tissue.
Transfer
the still wet precipitate to a piece of filter paper, which is put on a piece of
paper tissue. By doing this, most of the remaining rinse water is absorbed into
the paper tissue, allowing an even purer product. Do not put the precipitate
directly on paper tissue. Filter paper is much stronger and you can easily
scrape off the solid later on, but paper tissue becomes very weak when wet and
you will have a hard time recovering the precipitate when it is on weak and torn
apart paper tissue.
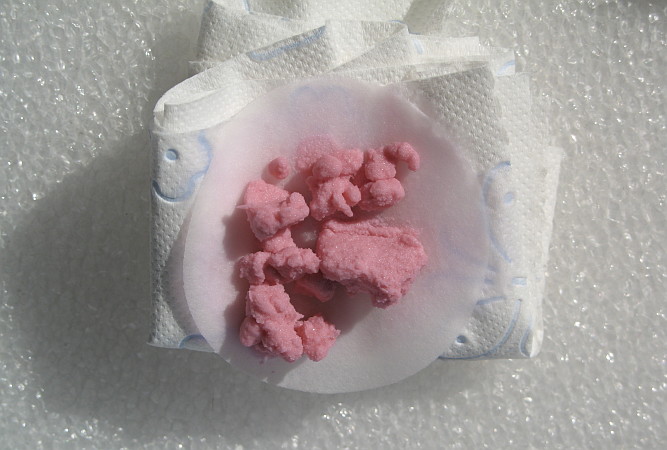
![]() When
most of the rinse water is absorbed by the paper tissue, then carefully scrape
the solid mass from the filter paper and transfer it to a petri dish. Put the
petri dish on a warm place (e.g. a heating radiator at 40 �C ... 60 �C) and
allow the solid to dry in a few hours. After a few hours spread the solid
somewhat more and allow it to dry for a few more hours. After half a day or so
you will have a completely dry, non-hygroscopic solid. The dry material
looks as follows in sunlight:
When
most of the rinse water is absorbed by the paper tissue, then carefully scrape
the solid mass from the filter paper and transfer it to a petri dish. Put the
petri dish on a warm place (e.g. a heating radiator at 40 �C ... 60 �C) and
allow the solid to dry in a few hours. After a few hours spread the solid
somewhat more and allow it to dry for a few more hours. After half a day or so
you will have a completely dry, non-hygroscopic solid. The dry material
looks as follows in sunlight:

![]() After
drying, the solid must be transferred to a clean and dry, tightly closed vessel.
The yield in this experiment was 5.1 gram. The two pictures below show the solid
in a little glass bottle. Left picture was made in daylight, right picture was
made under cold white TL light.
After
drying, the solid must be transferred to a clean and dry, tightly closed vessel.
The yield in this experiment was 5.1 gram. The two pictures below show the solid
in a little glass bottle. Left picture was made in daylight, right picture was
made under cold white TL light.


The original neodymium oxide, although of good quality, has some iron in it. The yellow/brown solution, obtained by dissolving the neodymium oxide in sulphuric acid (before the pink crystals separate from the liquid) has a weak, but definitely positive test on iron with thiocyanate. A solution of the pink crystals in distilled water is totally negative on testing with thiocyanate, even at high concentration of the lavender solution and thiocyanate. The dissolving of the pink crystals in water takes a long time!
If a few hundreds of mg of the pink crystals are taken and dissolved in water, then a lavender solution is obtained, very different from the yellow liquid, which remains after precipitation of the crystals. The following pictures nicely show the big difference. The top picture shows the liquids in daylight and the bottom picture shows the liquids under TL-light.


![]() The
yellow liquid can be used to obtain an additional amount of neodymium sulfate of
high purity, which tests negative on iron. Pour some of the liquid on a large
watch glass and put this watch glass in a warm place and allow most (but not
all!) of the liquid evaporate. Allow evaporation of approximately 80% of the
liquid. When this is done, then many nice pink/rose crystals are formed, which
easily can be separated from the remaining liquid.
The
yellow liquid can be used to obtain an additional amount of neodymium sulfate of
high purity, which tests negative on iron. Pour some of the liquid on a large
watch glass and put this watch glass in a warm place and allow most (but not
all!) of the liquid evaporate. Allow evaporation of approximately 80% of the
liquid. When this is done, then many nice pink/rose crystals are formed, which
easily can be separated from the remaining liquid.
Decant the liquid from the crystals by tilting the watch glass, such that the liquid flows away. Collect the liquid in a test tube and keep it. Scrape the crystals from the watch glass and rinse them two times with 2 ml of boiling hot distilled water, between rinses dapping them dry with a piece of filter paper.
![]() After the final rinse leave the crystals on a piece of filter
paper and allow them to dry in a warm place. This does not take a long time,
because these crystals already are fairly dry. After drying, transfer the
crystals to a small vial. The crystals have a beautiful pink/rose color and are
transparent.
After the final rinse leave the crystals on a piece of filter
paper and allow them to dry in a warm place. This does not take a long time,
because these crystals already are fairly dry. After drying, transfer the
crystals to a small vial. The crystals have a beautiful pink/rose color and are
transparent.

The liquid, decanted from the crystals has a fairly intense yellow color. When a drop of a concentrated solution of potassium thiocyanate is added to this yellow color, then a fairly strong red color appears. If a few of the crystals are crunched and dissolved in some distilled water and a drop of a concentrated solution of potassium thiocyanate is added to this, then no red color appears at all, the crystals have a negative test on iron. The iron is concentrated into the yellow liquid and is nicely separated from the neodymium sulfate. A solution of the crystals has a lavender color, exactly the same as a solution of the pink crystals, obtained after boiling the solution of neodymium oxide in dilute sulphuric acid.
![]()
Some additional tests
What is surprising is that neodymium oxide, when dissolved in sulphuric acid, leads to a yellow/brown solution in daylight or under TL-light. When the pink crystals are separated by boiling this solution, then the yellow/brown color even becomes stronger. What causes this strange behavior? Why don't you get a lavender solution?
Tests with the lavender solution
![]() The lavender solution in the round bottom flask as shown in the picture above
was used for further tests. This solution has a negative test for iron. To this
solution an excess amount of ammonia was added. This results in formation of a
pale lavender/blue precipitate. This precipitate was filtered on a fine filter
paper, using a vacuum pump (which still takes a lot of time, filtering is very
slow). The material was rinsed with distilled water while it was on the filter
to remove most of the ammonia and remaining sulfate ions. After the rinsing the
material was scraped from the filter and divided in two equal amounts.
The lavender solution in the round bottom flask as shown in the picture above
was used for further tests. This solution has a negative test for iron. To this
solution an excess amount of ammonia was added. This results in formation of a
pale lavender/blue precipitate. This precipitate was filtered on a fine filter
paper, using a vacuum pump (which still takes a lot of time, filtering is very
slow). The material was rinsed with distilled water while it was on the filter
to remove most of the ammonia and remaining sulfate ions. After the rinsing the
material was scraped from the filter and divided in two equal amounts.
![]() One half of the precipitate was added to 3 ml of dilute sulphuric acid. In
sulphuric acid, the material quickly dissolves without heating and the resulting
solution is pink/lavender in daylight, colorless under the light of a
fluorescent energy saving lamp and purely lavender in cold white TL light. Under
tungsten light the solution looks pink.
One half of the precipitate was added to 3 ml of dilute sulphuric acid. In
sulphuric acid, the material quickly dissolves without heating and the resulting
solution is pink/lavender in daylight, colorless under the light of a
fluorescent energy saving lamp and purely lavender in cold white TL light. Under
tungsten light the solution looks pink.
![]() The other half of the precipitate was transferred to a quartz tube and it was
heated with a roaring blue flame from a propane torch, such that the quartz tube
becomes red hot and starts glowing. After this treatment, a blue/grey powder was
obtained which is slightly darker than the original powdered neodymium oxide.
This blue/grey powder was allowed to cool down and then added to 3 ml of dilute
sulphuric acid. It only dissolves with difficulty and some heating is required
in order to dissolve it. The color of this solution is yellow under fluorescent
light of an energy saving lamp, grey/yellow under cold white TL light and
brown/pink in daylight.
The other half of the precipitate was transferred to a quartz tube and it was
heated with a roaring blue flame from a propane torch, such that the quartz tube
becomes red hot and starts glowing. After this treatment, a blue/grey powder was
obtained which is slightly darker than the original powdered neodymium oxide.
This blue/grey powder was allowed to cool down and then added to 3 ml of dilute
sulphuric acid. It only dissolves with difficulty and some heating is required
in order to dissolve it. The color of this solution is yellow under fluorescent
light of an energy saving lamp, grey/yellow under cold white TL light and
brown/pink in daylight.
Tests with the yellow/brown solution
![]() A small amount of the yellow/brown solution was not used for
making crystals of neodymium sulfate, but was set aside for another test. An
excess amount of ammonia was added to this solution, resulting in formation of a
white precipitate (less blue than the precipitate from the lavender solution).
The precipitate was not separated from the liquid.
A small amount of the yellow/brown solution was not used for
making crystals of neodymium sulfate, but was set aside for another test. An
excess amount of ammonia was added to this solution, resulting in formation of a
white precipitate (less blue than the precipitate from the lavender solution).
The precipitate was not separated from the liquid.
![]() Dilute
sulphuric acid was added drop-wise while swirling the test tube until the
solution is just clear again. In daylight this solution looks lavender, but
quickly it becomes opalescent again. When a few more drops of dilute sulphuric
acid were added a completely clear liquid was obtained. This liquid was grey in
TL-light and pale grey in daylight (all the additions of ammonia and sulphuric
acid made it rather dilute). The color is quite different from the original
yellow/brown solution. It looks more like a solution in dilute sulphuric acid
obtained from calcined neodymium oxide and probably it contains a mix of
lavender neodymium sulfate and the yellow/brown compound.
Dilute
sulphuric acid was added drop-wise while swirling the test tube until the
solution is just clear again. In daylight this solution looks lavender, but
quickly it becomes opalescent again. When a few more drops of dilute sulphuric
acid were added a completely clear liquid was obtained. This liquid was grey in
TL-light and pale grey in daylight (all the additions of ammonia and sulphuric
acid made it rather dilute). The color is quite different from the original
yellow/brown solution. It looks more like a solution in dilute sulphuric acid
obtained from calcined neodymium oxide and probably it contains a mix of
lavender neodymium sulfate and the yellow/brown compound.
![]()
Discussion of results
It seems that if calcined neodymium oxide is dissolved in dilute sulphuric acid, then a mix of two different kinds of neodymium sulfate is formed. One of them is lavender and the other is yellow/brown. A mix of these colors leads to brown/pink appearance in daylight or even grey appearance under TL light.
Apparently, the lavender kind of neodymium sulfate crystallizes when the liquid is heated to boiling, while the yellow/brown kind remains in solution. This explains why after separation of the pink crystal mass the liquid is really yellow/brown, even in daylight. Almost all ions, leading to lavender color have been separated in the pink crystalline mass.
If freshly precipitated neodymium hydroxide (made from a lavender solution) is dissolved in dilute sulphuric acid, then no yellow/brown sulfate is formed, the resulting solution is purely lavender again.
If the yellow/brown kind of neodymium sulfate is precipitated with ammonia and then redissolved in dilute sulphuric acid, then a mix of lavender and yellow/brown neodymium sulfate is obtained.
All these observations can be explained by assuming that neodymium sulfate can either be composed of free aqueous neodymium ions and free sulfate ions or of a sulfato-complex of neodymium.
- The free aqueous neodymium ions have a lavender color and this is the lavender compound, whose solubility strongly decreases with increasing temperature of the solution.
- The sulfate-complex has no free neodymium ions and the solubility of this compound does not decrease with increasing temperature of the solution. The complex is rather inert and ligand exchange (sulfato-aqua exchange) only occurs slowly. Precipitating as hydroxide partially destroys the sulfato-complex, explaining the observations with the test of the yellow/brown liquid.
Unfortunately no literature references are known to me about a yellow/brown sulfato-complex of neodymium.