


Neodymium ions -- narrow absorption bands
The color of an object depends on its absorption of visible light. Absorption is a property of an object and not of the light which falls on the object. Two kinds of absorption are relevant for the appearance of objects. All objects reflect light and some objects also transmit light. In both cases, not all light is reflected or transmitted. Some light is absorbed, the remaining light is reflected or transmitted.

The picture above shows transmission of light through liquids in test tubes and reflection of light from the background landscape, mainly green from grass.
If light of all wavelengths would be absorbed equally strong, then the appearance would be grey. The stronger the absorption, the darker the appearance of the object. If absorption of light depends on the wavelength of the reflected or transmitted light, then the object has a color different from grey. E.g. if a transparent liquid absorbs all blue light and violet light, but transmits green light and light of larger wavelength, then the liquid has a yellow appearance when one is looking through a layer of the liquid.
![]()
Some theoretical background
Light, as we know it is a mix of electromagnetic radiation of different wavelengths. With our eyes we only can see electromagnetic radiation in the wavelength range of just below 400 nm to just beyond 700 nm. Below 400 nm the sensitivity of the eye quickly diminishes and one goes into the so-called ultraviolet region and beyond 700 nm the visible red quickly fades for our eyes and one goes into the so-called infrared region. Some animals can see well beyond 400 nm, such as bees, which can see ultraviolet and there also are animals (some snakes) capable of seeing infrared light well beyond 700 nm.
Human perception of light
Below is a chart, which fairly well shows how we perceive light of a certain wavelength. If e.g. a light wave hits our eyes, which only contains light with a wave length of 550 nm, then we perceive this as green light. Light of 650 nm is perceived as red light, light of 450 nm is perceived as blue light.
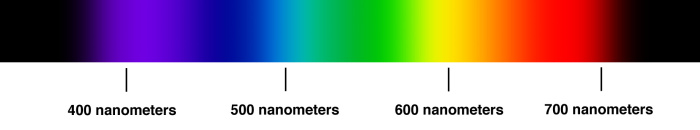
In real life, we hardly see any light containing electromagnetic radiation of a single wave length only. In such light, all objects only have shades of that single color. In reality, the light surrounding us is a mix of all visible wavelengths. When light of multiple wavelengths hits our eyes, then we perceive different colors. If e.g. light hits our eyes, which consists of approximately equal amounts of light of 550 nm and 650 nm, then we see a yellow color. So, our eye cannot distinguish between a beam of appr. 600 nm, which is perceived as yellow and a beam which is a mix of green and red light (550 nm and 650 nm). The picture below is a so-called colorwheel which shows how we perceive mixtures of pure red (650 nm), pure green (550 nm) and pure blue (450 nm).
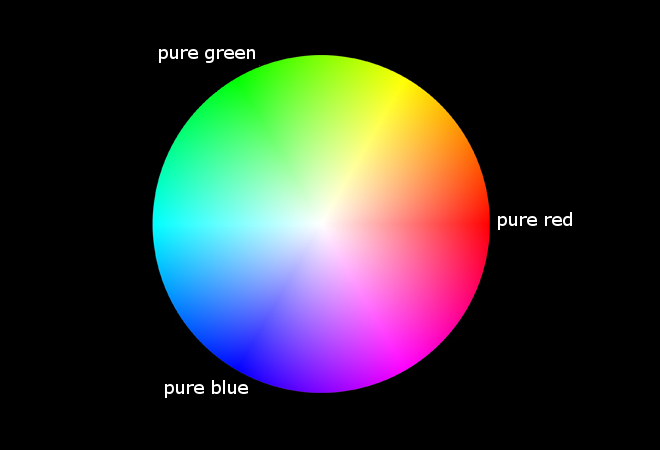
An equal mix of pure red, pure green and pure blue is perceived as white or a shade of grey (center of colorwheel). All colors from the visible spectrum can be mimiced with mixes of just these three colors. But one must be aware, that these colors are not true colors, they just look the same for us, because our brains do not distinguish between mixes of the primary colors (red, green and blue) and true colors from the visible spectrum. E.g. purple/indigo is around 400 nm in the visible spectrum, but a mix of blue and red (mainly blue, some red, see colorwheel above) can be made to look like purple/indigo, but this color mix does not contain any wavelengths below 450 nm. Only because of the properties of our brains we are able to mimic true colors by mixing other colors.
Absorption spectra of chemical compounds
For nearly all materials around us it is true that they have broad absorption spectra. E.g. a solution of copper sulfate absorbs light in the yellow and red regions and this makes the copper sulfate look blue. Now suppose that the copper sulfate is viewed in a light source, which does not cover the entire spectrum from 400 nm to 700 nm but only certain bands, such that the light source still looks white. The copper sulfate then still looks blue, although the hue may be somewhat different.
Now suppose there is some imaginary compound, which only absorbs light in narrow bands, and the light under which this compound is viewed has a banded spectrum. If the absorption bands are at frequencies which are not in the spectrum of the light source, then the compound does not absorb any light and it looks colorless under such a light source. The picture below makes this more clear.
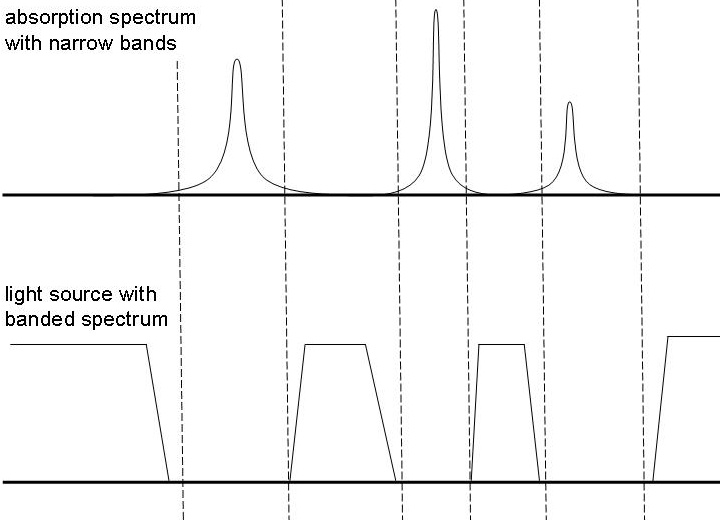
In a full spectrum, which has all frequencies in the visible range this compound certainly will have some color, but in the banded spectrum, as shown in the picture above, this compound seems colorless. So, this compound has the remarkable property that its color strongly depends on the light source under which it is viewed. It is exactly this type of compounds which are made in the experiment, described on this webpage.
![]()
![]() Required
chemicals:
Required
chemicals:
-
neodymium oxide of good purity (99.9% or better)
-
dilute sulphuric acid, 15% by weight
-
dilute nitric acid, 10% by weight
-
dilute hydrochloric acid, 10% by weight (high quality colorless acid is needed)
-
concentrated hydrochloric acid, 30% by weight (high quality colorless acid is needed, the yellow/green muriatic acid from a hardware store is not suitable)
![]() Required
equipment:
Required
equipment:
-
test tubes
-
means of heating a test tube carefully, e.g. an alcohol burner
![]() Safety:
Safety:
- The acids, used in this experiment are corrosive. Avoid contact with the skin, especially if the solutions are hot. If there is contact with the skin, then rinse with some cold water.
![]() Disposal:
Disposal:
- Only small amounts of neodymium are used and this metal only is slightly toxic. The waste can be flushed down the drain.
![]()
Preparation of the solutions
From a chemical point of view, this experiment is not difficult at all. A metal oxide is dissolved in some acids and the liquids are set aside for further observation.
![]() Take three test tubes and put a small spatula of neodymium oxide in each
test tube (around 200 mg of oxide in each test tube). Add 5 ml of dilute
sulphuric acid to one test tube, 5 ml of dilute nitric acid to the second test
tube and 5 ml of dilute hydrochloric acid to the third test tube. Most of the
oxide dissolves on adding the acid and some heat is produced (not much, but you
can feel some warming up of the test tubes).
Take three test tubes and put a small spatula of neodymium oxide in each
test tube (around 200 mg of oxide in each test tube). Add 5 ml of dilute
sulphuric acid to one test tube, 5 ml of dilute nitric acid to the second test
tube and 5 ml of dilute hydrochloric acid to the third test tube. Most of the
oxide dissolves on adding the acid and some heat is produced (not much, but you
can feel some warming up of the test tubes).
![]() Carefully heat all test tubes until all of the oxide has dissolved and a clear
liquid is obtained. With the sulphuric acid one must be careful not to heat too
much. Neodymium sulfate dissolves fairly well in cold water, but is almost
insoluble in hot water and getting it redissolved again is hard. So, be patient
when dissolving the neodymium oxide in the sulphuric acid. This problem does not
exist for the chloride and nitrate. Just keep on heating, until the oxide has
dissolved.
Carefully heat all test tubes until all of the oxide has dissolved and a clear
liquid is obtained. With the sulphuric acid one must be careful not to heat too
much. Neodymium sulfate dissolves fairly well in cold water, but is almost
insoluble in hot water and getting it redissolved again is hard. So, be patient
when dissolving the neodymium oxide in the sulphuric acid. This problem does not
exist for the chloride and nitrate. Just keep on heating, until the oxide has
dissolved.
Three liquids are obtained. Initially, the colors of the liquids look fairly strange for neodymium. The color of the solution in sulphuric acid seems to be brown/yellow and the color of the solution in nitric acid seems to be grey, but after standing for one day, all liquids have stabilized and all seem to have a pink or lavender color of some sort. The different initial color most likely is due to complex formation in the solution and might also due to very fine solid particles which need some more time to completely dissolve.
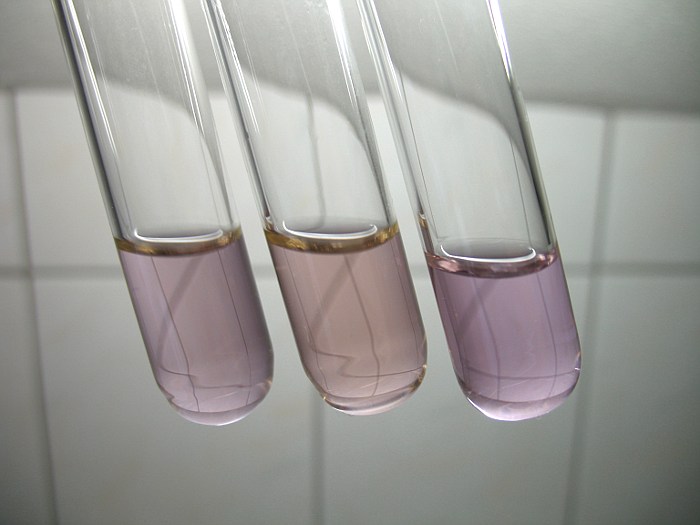
All three liquids still are strongly acidic, there was a large excess amount of acid while dissolving the neodymium oxide. Using such a large excess of acid assures that the liquids remain clear and no hydrolysis products are formed, such as insoluble basic neodymium salts.
![]()
Displaying the solutions under different light sources
For demonstration purposes, the liquids were transferred to three glass ampoules, which are sealed, such that a more permanent display is made of these liquids. Of course, when one just wants to do this experiment, the liquids can be kept in a test tube, but here it is decided to make a more permanent display. The ampoules were labeled, such that it remains clear which solution is in which ampoule. The appearance of the ampoules is very different under different light sources.
The picture below shows the solutions in daylight. Both the solution in sulphuric acid and the solution in hydrochloric acid look somewhat pink/rose, while the solution in nitric acid is more lavender.

When the liquids are viewed under fluorescent light from an energy saving lamp, then the result is totally different. The difference is really striking!

Now the solution in nitric acid is really colorless and the solutions in sulphuric acid and hydrochloric acid are pale yellow. Apparently the absorption bands of the neodymium ions, which give it a lavender color are at frequencies which are not in the output spectrum of the energy saving lamp. In the sulfate and chloride solutions there also is some absorption, which leads to a yellow color. This absorption is visible under the light of the energy saving lamp. This experiment now also explains why the sulfate and chloride solutions are pink, while the nitrate solution is lavender in daylight. The absorption, which leads to yellow light also is present in daylight and this mix of yellow color and lavender color apparently is perceived as pink color by the human brain.
Finally, when the liquids are viewed under the light of a cold-white TL tube, then there again is a different appearance.

Now the solution in sulphuric acid looks grey and the solution in nitric acid is even more lavender. It almost tends towards blue.
When the liquids are viewed under tungsten incandescent light, then they all look pink with a somewhat brown hue. The difference between the liquids then hardly is visible anymore. This also can be explained. Light from a tungsten incandescent bulb is mainly in the red to yellow part of the visible spectrum. Any absorption in the blue part of the spectrum (leading to yellow in the perceived appearance) is only weakly visible because tungsten light hardly contains any blue light. So, where in daylight there is a clear difference between pink and lavender solutions, in tungsten light this difference hardly is visible anymore.
Most striking, however, is the appearance under the light of a fluorescent energy saving lamp. This experiment also nicely demonstrates that the spectrum of such lamps is far from ideal and quite a lot of wavelengths are lacking. This is a well-known problem of energy saving lamps and for this reason many people still prefer the tungsten lamps, because these have a better continuous spectrum of emitted light.
Another experiment was done with neodymium oxide dissolved in concentrated hydrochloric acid. In the concentrated acid the solution looks quite different again. It is more lavender. Apparently the concentration of the chloride ion also has a strong effect on the appearance of neodymium ions in solution. This most likely is due to complex formation with the chloride. The solution in concentrated hydrochloric acid also was ampouled for a more permanent display, in a smaller ampoule. The pictures below show the solution in concentrated hydrochloric acid together with the solution in dilute hydrochloric acid, again under different light sources. The first picture is in daylight, the second picture in the light of a fluorescent energy saving lamp and the final picture is under cold white TL-light.



![]()
Different forms of neodymium sulfate
The above experiment shows the properties of neodymium ions when viewed under different light sources, but neodymium ions have more remarkable properties. The following is a preparation of pure neodymium sulfate from neodymium oxide and sulphuric acid, with the remarkable property that the method of preparation leads to two totally different compounds, but having very similar properties when in solution.
A separate webpage is made of this, it contains the procedure followed to make neodymium sulfate and it also highlights some not fully understood observations.
![]()
Discussion of results
![]() Neodymium ions in solution have very narrow absorption bands and this makes the
appearance of such solutions strongly dependent on the spectral properties of
the light under which such a solution is viewed. The absorption spectrum of a
solution of neodymium nitrate in dilute nitric acid looks as follows:
Neodymium ions in solution have very narrow absorption bands and this makes the
appearance of such solutions strongly dependent on the spectral properties of
the light under which such a solution is viewed. The absorption spectrum of a
solution of neodymium nitrate in dilute nitric acid looks as follows:
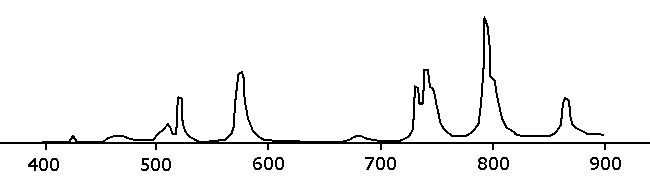
In the visible range there are two absorption peaks in the yellow and green region. This results in pink/lavender appearance in purely white light with a complete spectrum (look at the color wheel above and take the color, opposite to green/yellow). The fluorescent energy saving lamps apparently hardly emit light at the peaks of the absorption spectrum of neodymium ions.
![]() Besides that, the anion also has a strong influence on
the appearance of the neodymium ions. Although the lanthanide elements are said
to have much less pronounced color shifts due to complex formation, when
compared with the transition metal elements, the effect of a small shift is much
stronger. When a narrow absorption band is shifted somewhat then that will
hardly be noticed in daylight, but under a light source which has narrow bands
of wavelengths not present in its output spectrum there may be a large
difference. If a narrow absorption band shifts in or out of a band of absence of
light in the light source, then that can make a lot of difference in the
appearance of the solution.
Besides that, the anion also has a strong influence on
the appearance of the neodymium ions. Although the lanthanide elements are said
to have much less pronounced color shifts due to complex formation, when
compared with the transition metal elements, the effect of a small shift is much
stronger. When a narrow absorption band is shifted somewhat then that will
hardly be noticed in daylight, but under a light source which has narrow bands
of wavelengths not present in its output spectrum there may be a large
difference. If a narrow absorption band shifts in or out of a band of absence of
light in the light source, then that can make a lot of difference in the
appearance of the solution.