


Mini explosions -- high speed imaging
As shown in another experiment, when potassium permanganate and concentrated sulphuric acid are mixed, then an extremely sensitive and exceedingly strongly oxidizing compound is formed. This compound is manganese heptoxide, Mn2O7. For the practical setup of that experiment, one is referred to that other page. In this experiment, exactly the same chemical things were done, but images were made with a high speed digital camera, which takes 60 frames per second. Using that camera, beautiful new details are revealed and the experiment becomes really intriguing and remarkable.

 Manganese
heptoxide is a dark green oily compound, which is such a strong oxidizer that it
ignites any combustible material on first contact. Frequently, the material
explodes during this ignition. In this experiment a very small amount of this
compound is made and it is allowed to ignite some acetone. A very hot
and bright fire is produced, just by touching the liquid with a glass rod,
wetted with some acetone.
Manganese
heptoxide is a dark green oily compound, which is such a strong oxidizer that it
ignites any combustible material on first contact. Frequently, the material
explodes during this ignition. In this experiment a very small amount of this
compound is made and it is allowed to ignite some acetone. A very hot
and bright fire is produced, just by touching the liquid with a glass rod,
wetted with some acetone.
![]()
![]() Required
chemicals:
Required
chemicals:
-
concentrated sulphuric acid
-
potassium permanganate
-
acetone
![]() Required
equipment:
Required
equipment:
-
clean glass surface or petri dish
- glass rod
- high speed digital camera, 60 frames per second, with a tripod mount
- tripod, on which the camera should be mounted
![]() Safety
and disposal: See here.
Safety
and disposal: See here.
![]()
Procedure for performing the experiments
![]() The
procedure is the same for all experiments on this page, the only variations are
the lighting conditions and the amount of acetone, with which the glass rod is
wetted. The basic setup is described
here.
The
procedure is the same for all experiments on this page, the only variations are
the lighting conditions and the amount of acetone, with which the glass rod is
wetted. The basic setup is described
here.
For each experimental setup, the lighting conditions are mentioned in terms of aperture size and exposure time, which would be needed for a properly exposed standard image on a 24x36 mm negative film.
![]()
Daylight exposure: f/8, 1/400 second, 50 ASA
This is the first of a set of three experiments with bright daylight (sunny weather). This set of experiments nicely shows the shape of clouds of fire and smoke. This set of experiments does not really show the onset of the explosion itself, those phenomena are overwhelmed by the bright sunlight. These phenomena are described in another set of experiments with more dim light conditions.
The set of frames, shown below, are from a film, made by letting two drops of acetone fall on some Mn2O7. The series begins with a petri dish, with some Mn2O7 in it. The second image shows a drop of acetone, falling down. The third picture shows the same drop, somewhat lower and a smaller drop going down. The fourth picture shows the large drop, while it just touches the surface. In the fifth frame, one can see some purplish hue, where the drop hit the liquid and splashed apart. Just 16 ms later, the explosion already has produced a lot of light and then things quickly advance.
The complete sequence, shown below, consists of 28 frames and takes under half a second. When one is looking at the reaction, then one only sees a flash, a brief fire and a small cloud of smoke, and then all is over. The high-speed series really reveals a lot of beautiful detail of the reaction.
There was quite some wind, going from left to right. This causes the mushroom cloud of fire and smoke to be driven away to the right.


















This series also shows, that after the main explosion there are a few smaller explosions. Most notably is the explosion, shown in the next frame, which develops into a nice distorted cylindrical flame over time. The first frame of the series below this text is immediately after the last frame of the series above this text.










![]()
Daylight exposure: f/8, 1/400 second, 50 ASA
This experiment is very similar to the first one, also at daylight, with similar lighting conditions, but now, a very small amount of acetone is added, by dipping a wetted glass rod into the liquid. For the eye, the reaction was even less spectacular than the previous one, but the high speed captured images show a remarkable level of detail and formation of beautiful smoke clouds. Again, the wind from the left quickly blows the smoke away. These pictures show, that even in the bright lighting conditions, there is quite some overexposure. This means that the centre of explosion must be intensely hot and intensely bright. This is best demonstrated by the fourth picture.
The entire sequence shows that the actual violent reaction only takes approximately 10 frames. In terms of time, the total reaction takes between 150 ms and 200 ms, which is just a little more than the blink of an eye.

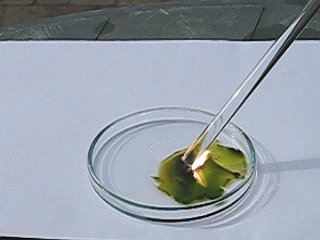
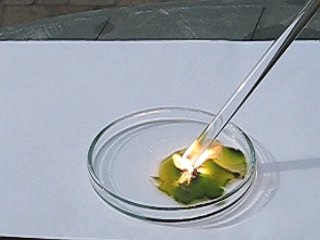



















A similar reaction was performed another time, at the same lighting conditions. To give an impression of the speed of the reaction, open the following two AVI files. One of these is in slow motion (10 times as slow as real time), the other is the same AVI as real time data. Size of these files is 388 KByte, download speed limited to 50 KByte per second.
slow motion AVI, 6 frames per second
real time AVI, 60 frames per second
![]()
Low-light conditions: f/4, 1/40 second, 100 ASA
An experiment is done, with low-light conditions, in order to get more insight in the onset of explosion. Just before the explosion starts, some really peculiar light effects can be observed, which only can be captured at low light. The explosion itself is totally over-exposed, but the onset of explosion shows some very interesting features.
Below, a high-resolution sequence is shown, taken from a movie at 30 frames per second, at a resolution of 640x480 pixels. In this experiment a single drop of acetone is dripped onto the Mn2O7.
The first two pictures show the situation, just before the drop is in the petri dish, and just after the drop falls in the petri dish. The drop on the glass can be clearly observed. There also is some purple light. I have no true explanation for this strange purple light. It is the onset of explosion. This purple does not show up in every sequence, captured by the camera. Most of the times, it probably occurs in the same time frame as the bright explosion itself, and then (if this effect is present at all) it will drown in the over-exposure due to the bright light of the explosion. The next 2 frames show the explosion itself. These frames are strongly over-exposed. The intensity of the light is very high, compared to the intensity of the ambient light. The bright light lasts for only two frames (at most 66 ms).






![]()
Medium-light conditions: f/2.8, 1/125 second, 50 ASA
The following is a sequence of images, captured with medium light conditions. Multiple experiments were performed under these light conditions. Also here, one of the experiments shows the strange purple light, and that sequence is shown here. This sequence is taken at 60 frames per second.


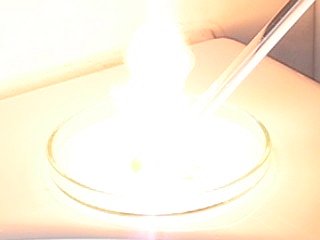

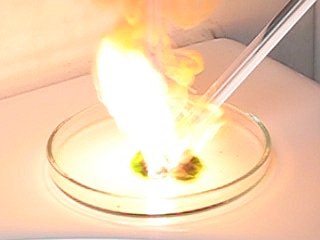
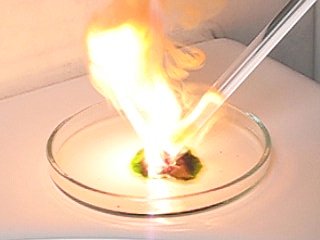






Now, the question is, what is that purple cloud-like phenomenon, at the onset of explosion? It can be captured approximately 1 out of 3 experiments. If it occurs, it always appears in the frame, just before the very bright explosion. It cannot be flares in the lens of the camera, because the 640x480 image with that phenomenon has no bright lights in the image. Is it some UV-light, which first is emitted, before the explosion really occurs?
What is remarkable of this phenomenon, is, that if it occurs, it always appears from the center of explosion to the bottom part of the image around an almost straight line. That would indicate some optical effect, but the strange thing is that it also occurs without any bright light in the image. Maybe it is a flare-effect, but at the UV-part of the spectrum.