Liquid from hell -- fire on first contact
When potassium permanganate and concentrated sulphuric acid are mixed, then an extremely sensitive and strongly oxidizing compound is formed. This compound is manganese heptoxide, Mn2O7.

 Manganese
heptoxide is a dark green oily compound, which is such a strong oxidizer that it
ignites any combustible material on first contact. Frequently, the material
explodes during this ignition. In this experiment a very small amount of this
compound is made and it is allowed to ignite some combustible liquid. A very hot
and bright fire is produced, just by touching the liquid with a glass rod, on
which some combustible material is present. Any combustible material is
suitable, e.g. paper, organic solvents, dust, hair, etc. The safest way,
however, for doing this experiment is with a combustible liquid. When small
pieces of paper are used, then a small but very violent explosion frequently
occurs, in which zillions of small droplets of acid are sprayed around.
Manganese
heptoxide is a dark green oily compound, which is such a strong oxidizer that it
ignites any combustible material on first contact. Frequently, the material
explodes during this ignition. In this experiment a very small amount of this
compound is made and it is allowed to ignite some combustible liquid. A very hot
and bright fire is produced, just by touching the liquid with a glass rod, on
which some combustible material is present. Any combustible material is
suitable, e.g. paper, organic solvents, dust, hair, etc. The safest way,
however, for doing this experiment is with a combustible liquid. When small
pieces of paper are used, then a small but very violent explosion frequently
occurs, in which zillions of small droplets of acid are sprayed around.
![]()
![]() Required
chemicals:
Required
chemicals:
-
concentrated sulphuric acid
-
potassium permanganate
-
acetone or any other flammable liquid (e.g. ethanol, methanol)
-
optionally: charcoal, glycerol, sulphur
![]() Required
equipment:
Required
equipment:
-
clean glass surface or petri dish
- glass rod
![]() Safety:
Safety:
-
 Do not scale up
this experiment! The material, made in this experiment is exceedingly
dangerous. With the quantities, used here, the risk is not that large, but
with larger quantities there is an unacceptable risk of severe injury, fire
or explosion!
Do not scale up
this experiment! The material, made in this experiment is exceedingly
dangerous. With the quantities, used here, the risk is not that large, but
with larger quantities there is an unacceptable risk of severe injury, fire
or explosion! -
 Do not store
any of the material, made in this experiment. It is unstable and
may explode, apparently without reason. Make a small quantity and use
it immediately for small experiments.
Do not store
any of the material, made in this experiment. It is unstable and
may explode, apparently without reason. Make a small quantity and use
it immediately for small experiments. -

 The material
formed in this experiment also is exceedingly corrosive. A drop of this
material on your skin will result in instant hot burning of your skin. NEVER EVER ALLOW ANY DROP OF
THIS MATERIAL TO TOUCH YOUR SKIN!!
The material
formed in this experiment also is exceedingly corrosive. A drop of this
material on your skin will result in instant hot burning of your skin. NEVER EVER ALLOW ANY DROP OF
THIS MATERIAL TO TOUCH YOUR SKIN!! - Concentrated sulphuric acid is very corrosive, do not allow this acid to come in contact with the skin. In case of an accidental spill, immediately wipe off with a dry tissue and then at once rinse with a lot of cold water.
- Potassium permanganate is a strong oxidizer.
- An open flame is produced in this experiment, so be sure that no open bottles with volatile flammable liquids are around.
![]() Disposal:
Disposal:
- The amount of waste is so small, that it can be rinsed down the drain with a lot of water. Be careful when rinsing away the green liquid. If brown stains of partially reacted potassium permanganate remain, then a mix of dilute 5% hydrochloric acid and a sulfite or metabisulfite, or a mix of dilute 5% hydrochloric acid and 3% hydrogen peroxide can be used to remove the permanganate stains. Also stains on skin can be treated with such a mix, but the skin should be rinsed quickly after removal of the stains.
![]()
Procedure for performing the experiment
![]() Take a
very small amount of potassium permanganate (a really small pinch at the end of
a spatula), somewhere between 10 and 20 mg. Do not use larger quantities.
Take a
very small amount of potassium permanganate (a really small pinch at the end of
a spatula), somewhere between 10 and 20 mg. Do not use larger quantities.
![]() Put a
few drops of concentrated sulphuric acid on the potassium permanganate, such
that all solid is covered. Do not use too much acid. That might make the
permanganate too dilute.
Put a
few drops of concentrated sulphuric acid on the potassium permanganate, such
that all solid is covered. Do not use too much acid. That might make the
permanganate too dilute.
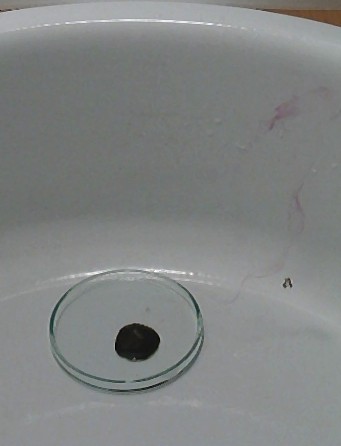
![]() Let the
acid act on the solid potassium permanganate for half a minute. A dark green
liquid is formed, with a somewhat oily appearance.
Let the
acid act on the solid potassium permanganate for half a minute. A dark green
liquid is formed, with a somewhat oily appearance.
![]() Take a glass rod and put a single drop of acetone or other
combustible liquid on the tip. This can be done easily by putting some of the
liquid in a small test tube (0.5 ml or so) and keeping it almost horizontally.
Then put the glass rod in the liquid, such that quite a large part of it is
wetted. Then take it out of the test tube and keep it at an angle of around 45
degrees. A nice small drop collects at the tip of the rod. Carefully touch the
dark green liquid with the tip of the rod.
Take a glass rod and put a single drop of acetone or other
combustible liquid on the tip. This can be done easily by putting some of the
liquid in a small test tube (0.5 ml or so) and keeping it almost horizontally.
Then put the glass rod in the liquid, such that quite a large part of it is
wetted. Then take it out of the test tube and keep it at an angle of around 45
degrees. A nice small drop collects at the tip of the rod. Carefully touch the
dark green liquid with the tip of the rod.
The result of this experiment is shown in the pictures below. As soon as the combustible liquid touches the green oily liquid, a violent reaction starts, with a bright fire. It is amazing to see such a large and bright flame from such a tiny amount of acetone. Sometimes, the experiment is accompanied by a small explosion.
Below, 6 frames of a small movie are shown. The total time, spanned by these 6 images is only ⅓ of a second, so the intense bright flash only lasts for 150 ms.






Two movies can be downloaded and viewed of the reaction. Each movie has a size of approximately 1 MByte: movie 1 and movie 2.
Another special phenomenon is the color of the smoke, produced during the reaction. Initially, brown smoke is produced. This brown smoke is mostly MnO2. After a while, when no combustible liquid remains, but the manganese heptoxide still is quite warm, the color of vapor of Mn2O7 can be observed. This compound is volatile and its vapor is purple, as shown in the picture below. By clicking the picture, a slow-motion movie can be downloaded.
After the reaction, a dark green liquid remains, and around the liquid, there is a purple rim. This purple rim is formed due to volatilization of Mn2O7 and settling of the compound on the glass again, around the dark spot. The compound looks red, when viewed through a very thin layer. It looks green, when present in thicker layers.

![]()
Results with charcoal, glycerol and sulphur powder
When this experiment is done with a small piece of charcoal, then as soon as it touches the liquid, a sharp crackling noise is heard and a flash can be observed in the liquid. Unfortunately this is not very spectacular, when viewed in an animation, but nevertheless, the small movie demonstrating this can be viewed here.
With glycerol the result is more spectacular. There is some crackling, flashing, and a lot of brown smoke. The result can be viewed here.
With sulphur, no reaction occurred. This does not mean that indeed no reaction can occur. The mix with sulphur can be insidiously dangerous, because it can set off at any moment, when it is not expected. However, in this experiment, no visible reaction with flowers of sulphur could be observed.
![]()
Discussion of results
![]() Potassium permanganate reacts with concentrated sulphuric acid as follows,
manganese heptoxide being formed in the reaction:
Potassium permanganate reacts with concentrated sulphuric acid as follows,
manganese heptoxide being formed in the reaction:
2KMnO4 + 2H2SO4 → Mn2O7 + 2KHSO4 + H2O
The green color is due to dissolved Mn2O7 in concentrated sulphuric acid. A very thin layer of this compound looks red/purple and the vapor of this compound is purple.
![]() Manganese heptoxide is very unstable. It slowly decomposes, giving MnO2
and highly active oxygen. A simplified balanced equation is given below:
Manganese heptoxide is very unstable. It slowly decomposes, giving MnO2
and highly active oxygen. A simplified balanced equation is given below:
Mn2O7 → 2MnO2 + 3[O]
When no combustible compounds are present, then plain oxygen and ozone are formed very slowly. When a combustible compound is present, then the released active oxygen is capable of igniting the combustible material at once. This reaction is very violent and often leads to explosive decomposition of remaining manganese heptoxide.
The brown smoke, released in the beginning of the experiment is finely divided MnO2, in a later stage, when the material cools down somewhat, it does not decompose anymore and the purple vapor of Mn2O7 can be observed.