


Nickel - ethylenediamine complexes
Ethylenediamine, NH2CH2CH2NH2 is a well-known ligand for many transition metals. It is a bidentate ligand, capable of coordination on both nitrogen atoms. In this webpage, the different complexes with nickel(II) are demonstrated.

One of the complexes is isolated in the form of the perchlorate salt. This complex has a nice bright color and also is an energetic compound.
In another very similar experiment, the copper complex is made as well.
![]()
![]() Required
chemicals:
Required
chemicals:
- nickel sulfate
- ethylenediamine
- nickel nitrate (optional, for second experiment only)
- ammonium perchlorate (optional, for second experiment only)
- nickel carbonate (optional, for third experiment only)
- perchloric acid (optional, for third experiment only)
![]() Required
equipment:
Required
equipment:
- test tubes
- petri dish
- small burner
- filter paper (optional, for second experiment only)
![]() Safety:
Safety:
- Nickel salts are carcinogenic. Repeated exposure to nickel(II) salts may lead to allergic reactions, in some people, nickel is a skin sensitizer.
- Ethylenediamine is quite a strong base, hence it is corrosive. This compound is intensely strongly fuming and has a smell reminiscent to the smell of ammonia (although it is less pungent). Avoid contact with skin and avoid inhaling the fumes.
- Perchlorate is a strong oxidizer and in contact with fuels it promotes fire. The complex, produced in this experiment, is an energetic compound.
![]() Disposal:
Disposal:
- Nickel salts should not go down the drain. Bring the waste solutions which contain nickel(II) salts to a suitable municipal waste processing facility. Remains of ethylenediamine and ammonium perchlorate can be dissolved in water and flushed down the drain with lots of water.
![]()
The nickel(II) complexes of ethylenediamine
In this experiment, the gradual displacement of water ligands by ethylenediamine is demonstrated and it is shown what colors these complexes have.
Ethylenediamine first is dissolved in water. This makes working with the compound less annoying. Pure ethylenediamine gives off intensely strong fumes, as shown by the picture below.

For the purpose of this experiment, the ethylenediamine is diluted, such that a solution of approximately 15% by weight is obtained. Such a dilute solution does not fume at all.
In the experiment, some nickel(II) sulfate is dissolved in water and in small steps, solution of ethylenediamine is added. After each addition, the test tube, containing the reagents, is swirled around, such that all liquid is well mixed. The two pictures below show a test tube, filled with a solution of nickel(II) sulfate and the result of adding a small amount of the 15% solution of ethylenediamine. The right picture nicely shows the different stages of coordination to ethylenediamine.


When the test tube in the right picture above is swirled, then a cyan solution is obtained, which contains a mix of plain nickel(II) and a complex of ethylenediamine and nickel(II). A few more drops of the solution of ethylenediamine need to be added to have (nearly) all nickel(II) converted to a complex. The result is as in the left picture below:


The complex has a bright light blue color, somewhat like the color of plain aqueous copper(II) ions, such as in solutions of copper sulfate, but somewhat lighter. In this complex, there is one molecule of ethylenediamine for each nickel(II) ion. When more ethylenediamine is added, as shown in the right picture, then a much darker blue complex is formed.
A better demonstration of the much darker compound is given below. The two pictures below show the dark blue complex which has 2 molecules of ethylenediamine for each nickel(II) ion. The dark blue compound was prepared by carefully adding a few more drops of ethylenediamine and shaking, such that a homegeneous royal blue solution is obtained. When even more ethylenediamine is added, then a violet complex is formed. This is shown in the right picture below.


On addition of a little more ethylenediamine and shaking, a homogeneous beautiful purple/violet solution is obtained.
The violet complex is soluble in water, but apparently, the solution shown below is over-saturated. Part of the solution was transferred to another test tube and set aside. One day later, a large part of the complex has settled at the bottom as a crystalline complex. The liquid is much lighter.


The solid material at the bottom of the test tube can be isolated and dried. The precipitate, however, is quite voluminous and contains a lot of water, although the picture looks otherwise. After drying only a very small amount of fine crumbs remains.
The ethylenediamine complexes of nickel are quite stable in neutral and alkaline solutions, but they cannot exist in acidic solution. Part of the purple solution, as shown above, was added to some dilute sulphuric acid. When this is done, the complex is destroyed at once and a plain green solution, containing hydrated nickel(II) ions is obtained.
Preparation of a solid complex
Better results for isolation of the complex are obtained when the perchlorate salt is prepared. Preparing the perchlorate at high purity is not easily done with nickel sulfate (the sulfate easily crystallizes together with the perchlorate), but it works very well, when one starts from nickel nitrate (the nitrate is much more soluble than the sulfate, while the perchlorate is less soluble). The procedure is as follows:
![]() Prepare
a fairly concentrated solution of nickel(II) nitrate. This can be done easily,
nickel(II) nitrate is very soluble in water.
Prepare
a fairly concentrated solution of nickel(II) nitrate. This can be done easily,
nickel(II) nitrate is very soluble in water.
![]() Add
drops of an approximately 50% solution of ethylenediamine (much less fuming
than the pure chemical) and swirl after adding each drop and continue until a
purely violet solution is obtained. Then add one additional drop of
ethylenediamine.
Add
drops of an approximately 50% solution of ethylenediamine (much less fuming
than the pure chemical) and swirl after adding each drop and continue until a
purely violet solution is obtained. Then add one additional drop of
ethylenediamine.
![]() In a
separate test tube dissolve an excess amount of ammonium perchlorate in hot
water.
In a
separate test tube dissolve an excess amount of ammonium perchlorate in hot
water.
![]() Add both
solutions to each other and allow the liquid to cool down. When this is done,
then slowly a nice crystalline mass is formed and crystals settle at the bottom
of the liquid. These crystals have a size of a few tenths of a mm (e.g. like
table salt). It takes a few tens of minutes before a decent amount of crystals
has separated from the liquid. Decant the liquid above the crystals as good as
possible from the crystal mass. This can be done easily, the crystal mass is
compact.
Add both
solutions to each other and allow the liquid to cool down. When this is done,
then slowly a nice crystalline mass is formed and crystals settle at the bottom
of the liquid. These crystals have a size of a few tenths of a mm (e.g. like
table salt). It takes a few tens of minutes before a decent amount of crystals
has separated from the liquid. Decant the liquid above the crystals as good as
possible from the crystal mass. This can be done easily, the crystal mass is
compact.
![]() Take a
pile of paper tissues and put a piece of filter paper on that. On the filter
paper put the crystal mass and carefully press this between a pile of paper
tissue. Repeat this process until no more humidity comes from the crystal mass.
In this way, a fairly dry crystal mass can be isolated.
Take a
pile of paper tissues and put a piece of filter paper on that. On the filter
paper put the crystal mass and carefully press this between a pile of paper
tissue. Repeat this process until no more humidity comes from the crystal mass.
In this way, a fairly dry crystal mass can be isolated.
![]() Put the
crystal mass in a petri dish and put aside for half a day or so in a dry and
warm place, free of dust. Spread the solid material somewhat every few hours to
allow all of it to dry well.
Put the
crystal mass in a petri dish and put aside for half a day or so in a dry and
warm place, free of dust. Spread the solid material somewhat every few hours to
allow all of it to dry well.
The final result of the process as outlined above is a nice perfectly dry violet solid.

The picture above shows a sample of well over 1 gram of the solid.
This solid is soluble in water, but not very easily. Its solubility is better than the solubility of potassium perchlorate, but it is much less soluble than ammonium perchlorate or nickel nitrate. Solutions of this solid are light violet.
![]()
Failed attempt to isolate the blue bis(ethylenediamine) bi aqua nickel(II) complex
![]() In this experiment, an excess amount of nickel(II) carbonate
was added to an approximately 15% by weight solution of perchloric acid. After
the initial fizzling, the solution was heated for quite some time to assure that
all perchloric acid is consumed. The result is a turbid liquid, which has a high
concentration of nickel perchlorate, with finely suspended solid nickel
carbonate in it.
In this experiment, an excess amount of nickel(II) carbonate
was added to an approximately 15% by weight solution of perchloric acid. After
the initial fizzling, the solution was heated for quite some time to assure that
all perchloric acid is consumed. The result is a turbid liquid, which has a high
concentration of nickel perchlorate, with finely suspended solid nickel
carbonate in it.
![]() The
solution was poured in a thin test tube which was put aside for two days, with a
rubber stopper on it to assure no dust could enter the test tube. After two days
there was a perfectly clear bright green solution on top of a thin layer of pale
green precipitate (unreacted remains of the nickel carbonate). Using a pasteur
pipette with a long needle-like tip, the clear liquid is sucked away from the
precipitate and transferred to a separate test tube.
The
solution was poured in a thin test tube which was put aside for two days, with a
rubber stopper on it to assure no dust could enter the test tube. After two days
there was a perfectly clear bright green solution on top of a thin layer of pale
green precipitate (unreacted remains of the nickel carbonate). Using a pasteur
pipette with a long needle-like tip, the clear liquid is sucked away from the
precipitate and transferred to a separate test tube.
![]() Drops of
a 25% solution of ethylenediamine are added to the bright green liquid, until
the liquid has a deep blue color. After each drop the liquid is shaked to assure
good mixing. Addition of drops does not lead to formation of precipitates. First
the liquid becomes cyan, then light blue like dilute copper sulfate and then the
liquid darkens after addition of each drop. As soon as a single drop did not
cause any visible deepening of the blue color, addition of ethylenediamine was
stopped. The end result was a beautiful deep blue liquid, much like the liquid,
shown in one of the pictures above.
Drops of
a 25% solution of ethylenediamine are added to the bright green liquid, until
the liquid has a deep blue color. After each drop the liquid is shaked to assure
good mixing. Addition of drops does not lead to formation of precipitates. First
the liquid becomes cyan, then light blue like dilute copper sulfate and then the
liquid darkens after addition of each drop. As soon as a single drop did not
cause any visible deepening of the blue color, addition of ethylenediamine was
stopped. The end result was a beautiful deep blue liquid, much like the liquid,
shown in one of the pictures above.
![]() The deep
blue solution was transferred to a petri dish and put in a warm dry place, free
of dust. After one day, the volume of liquid was less than when it was made and
the color of the liquid still was deep blue. After two days, all liquid had
evaporated, but no nice dry crystalline mass was produced. A dirty-looking, very
sticky, green/brown paste was sticking to the glass of the petri dish. After yet
another half a day, the sticky tar-like material still was tar-like (like a
nearly dry syrup, which does not dry further). It could be scraped off, but it
remained sticking to the spatula.
The deep
blue solution was transferred to a petri dish and put in a warm dry place, free
of dust. After one day, the volume of liquid was less than when it was made and
the color of the liquid still was deep blue. After two days, all liquid had
evaporated, but no nice dry crystalline mass was produced. A dirty-looking, very
sticky, green/brown paste was sticking to the glass of the petri dish. After yet
another half a day, the sticky tar-like material still was tar-like (like a
nearly dry syrup, which does not dry further). It could be scraped off, but it
remained sticking to the spatula.
![]() The dark
green/brown paste was not kept around, some water was added to it to see whether
it can be reverted to the nice deep blue solution. This is not the case. The
paste does dissolve fairly easily, but it gives a turbid pale blue liquid.
Probably the pale liquid contains some of the bis-ethylenediamine complex, but
also a lot of nickel hydroxide.
The dark
green/brown paste was not kept around, some water was added to it to see whether
it can be reverted to the nice deep blue solution. This is not the case. The
paste does dissolve fairly easily, but it gives a turbid pale blue liquid.
Probably the pale liquid contains some of the bis-ethylenediamine complex, but
also a lot of nickel hydroxide.
The isolation of the complex did not succeed. A small excess amount of ethylenediamine was added to the turbid pale blue liquid and this immediately caused formation of a very fine purple precipitate and formation of the violet liquid. This liquid was heated and even gentle heating was sufficient to make it completely clear and deep violet. On cooling down, many nice glittering violet crystals were produced of tris(ethylenediamine) nickel(II) perchlorate. The liquid above these crystals was decanted and these crystals were dried in the same way as described above.
So, it can be concluded that the tris-complex can be isolated perfectly well, but the bis-complex cannot easily be isolated. Most likely this is due to the more labile properties of the complex and partial loss of ethylenediamine from the liquid when it is allowed to evaporate to dryness.
![]()
Heating of tris(ethylenediamine) nickel(II) perchlorate
When the solid material is heated in a flame, then it explodes with great violence, producing a strong hissing/crackling sound. The following four pictures show a tiny sample on the tip of a miniature spatula. At the top right, the deflagration of part of the material is shown. One somewhat larger crystal remains on the spatula and only at most 67 ms later, that crystal also deflagrates. In real time, the burning of this material is with strong crackling and a somewhat stroboscopic effect.




![]()
Discussion of results
![]() Ethylenediamine coordinates to many metal ions through both of its nitrogen
atoms. It has free electron pairs on each of the nitrogen atoms. The
coordination looks as follows:
Ethylenediamine coordinates to many metal ions through both of its nitrogen
atoms. It has free electron pairs on each of the nitrogen atoms. The
coordination looks as follows:
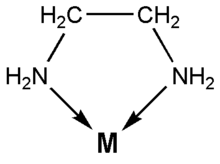
Here M is the metal ion and the arrows indicate the sharing of the free electron pairs of the nitrogen with the metal ion. In this experiment, M is the nickel(II) ion, Ni2+.
![]() Nickel can (and usually does) coordinate to six atoms with a free electron pair.
Fully aquated nickel ions has six water molecules coordinated to it as shown
below.
Nickel can (and usually does) coordinate to six atoms with a free electron pair.
Fully aquated nickel ions has six water molecules coordinated to it as shown
below.
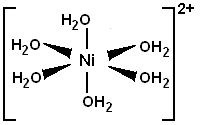
Water molecules can be replaced by ethylenediamine molecules. Each two water molecules are replaced by one ethylenediamine molecule.
This results in the following 4 complexes (en is a shorthand notation for ethylenediamine):
| [Ni(OH2)6]2+ |
 |
| [Ni(en)(OH2)4]2+ |
 |
| [Ni(en)2(OH2)2]2+ |
 |
| [Ni(en)3]2+ |
 |
![]() The perchlorate salt of the fourth complex ion only is
moderately soluble and easily is isolated in solid form. The purple compound
hence can be written as
The perchlorate salt of the fourth complex ion only is
moderately soluble and easily is isolated in solid form. The purple compound
hence can be written as
[Ni(en)3](ClO4)2
This perchlorate salt is an energetic compound. It carries its own fuel and oxidizer. The ethylenediamine ligands are a perfect fuel for the perchlorate oxidizer. The oxygen balance is not perfect, but it is close enough to make the compound energetic and fairly easily ignited.
![]() Ethylenediamine is a fairly strong base and is easily protonated by acids, even
if the ligand is coordinated to a metal ion:
Ethylenediamine is a fairly strong base and is easily protonated by acids, even
if the ligand is coordinated to a metal ion:
NH2CH2CH2NH2 + 2H+ → +NH3CH2CH2NH3+
Both amine-groups are protonated by acid and the free electron pair on each of the nitrogen atoms then coordinates to the H+-ion instead of the metal-ion. The ligand then loses contact with the metal ion and the metal ion is coordinated by water again.