


Copper - ethylenediamine complex
In this page, it is demonstrated how ethylenediamine combines with copper(II) ions to form a very dark blue/purple complex. This complex can also be isolated in crystalline form, just like the nickel-complex.

The copper complex is isolated as the perchlorate salt. It is demonstrated that this is an energetic compound.
![]()
![]() Required
chemicals:
Required
chemicals:
- copper(II) oxide
- ethylenediamine
- perchloric acid (30%)
![]() Required
equipment:
Required
equipment:
- test tubes
- small erlenmeyer
- petri dish
- small burner
- filter paper
![]() Safety:
Safety:
- Ethylenediamine is quite a strong base, hence it is corrosive. This compound is intensely strongly fuming and has a smell reminiscent to the smell of ammonia (although it is less pungent). Avoid contact with skin and avoid inhaling the fumes.
- Perchlorate is a strong oxidizer and in contact with fuels it promotes fire. The complex, produced in this experiment, is an energetic compound.
- Perchloric acid is corrosive. In this experiment, however, only relatively dilute perchloric acid is used and this is not more dangerous than hydrochloric acid of similar concentration.
![]() Disposal:
Disposal:
- Copper salts should not go down the drain. Bring the waste solutions which contain copper(II) salts to a suitable municipal waste processing facility. Remains of ethylenediamine and perchloric acid can be mixed with a lot of water and may be flushed down the drain.
![]()
Preparation of a solution of copper(II) perchlorate
In this experiment a fairly concentrated solution of copper(II) perchlorate is needed. This chemical is not easily obtained commercially, hence it is prepared in solution. Isolation of this chemical in solid form is not necessary.
The production of a solution of copper(II) perchlorate is done by dissolving copper(II) oxide in dilute perchloric acid. The picture below shows a standard 16 mm diameter test tube with some copper(II) oxide in it.

Copper(II) oxide is a very compact chemical with high density, so quite a lot of dilute perchloric acid is needed to dissolve the shown amount. A solution of 30% perchloric acid is added to the solid copper(II) oxide. When this is done, then there is slight heating of the liquid (it becomes luke-warm). It is important to add the acid in small steps and each time assure that there still is an excess amount of CuO. The two pictures below show a bright blue solution with excess perchloric acid in solution on the left (with some CuO on the bottom) and on the right there is a solution with excess CuO, which is more dull blue and somewhat turbid.


Keep on adding perchloric acid in small amounts and each time when the liquid is clear and bright blue, shake and heat the liquid to near boiling to get more CuO dissolved. Stop adding perchloric acid, when the liquid is turbid and dull blue, but hardly any solid CuO is left at the bottom of the test tube.
At the end, it is best to have a solution with copper perchlorate, in which there is slight excess amount of CuO. The pictures also show how much acid is needed to get all CuO dissolved.
Preparation of the copper complex of ethylenediamine
When the solution of copper perchlorate is ready, then transfer it to a small erlenmeyer. Allow it to cool down somewhat and then add ethylenediamine to the solution. You can add the pure chemical, but it is better to mix the chemical with some water first, before it is added to the solution of copper perchlorate.
Initially, the liquid turns very dark blue when not too much ethylenediamine is added and the liquid is swirled:

This dark blue liquid most likely contains a copper complex in which the water-ligands of the copper ions are partially replaced by ethylenediamine. This blue complex, however, is not very stable. On heating or on long standing, a light blue solid separates from this dark blue liquid. The light blue solid most likely is a basic copper perchlorate, or maybe even copper hydroxide. It certainly is not an ethylenediamine complex of copper.
Next, more ethylenediamine (a mix of 1 part of ethylenediamine to 3 parts of water is suitable) is added in small steps, until the liquid has become much more purple. When the color does not change anymore on adding more ethylenediamine, then just to be sure add a little bit more (e.g. 1 ml of the ethylenediamine/water mix). When this is done, then all solid matter which still was present in the blue liquid will certainly dissolve and a clear and very dark purple/blue liquid is obtained. This liquid has excess ethylenediamine in solution.

Heat this dark purple/blue liquid, until it boils. No solid matter may remain present in the boiling hot liquid. If there is solid matter, then add another milliliter of the mix of ethylenediamine and water and boil again for a while.
Put the clear liquid aside in a dust-free place and let it stand for several hours. A lot of solid material will be present at the bottom of the erlenmeyer. Put the liquid (with the solid material in it) in a freezer and let it cool down to 0 ºC or even a little colder. The liquid, however, must not become frozen.
The ice cold liquid must be decanted from the solid material. A remarkably large amount of solid crystalline matter will be present at the bottom of the erlenmeyer. Put this wet solid material on a filter paper, which in turn is put on a pile of paper tissue:

Carefully scrape the solid to the center of the filter paper and then fold the filter paper, such that the solid is inside. Fold the tissue paper around the filter paper and firmly press the tissue paper with the filter paper inside, holding the dark solid material. Be sure that the dark solid does not come in direct contact with the tissue paper. If this happens, then it will be very hard to separate the crystalline solid from the tissue paper.
When the tissue paper has absorbed a lot of purple/blue liquid, then replace it with fresh tissue paper and again firmly press the filter with the crystalline solid in it between the tissue paper. After three rounds the crystalline solid only is damp, nearly all liquid is absorbed by paper tissue. Spread the damp solid on the filter paper.

The damp solid must be put in a warm and dry place for half a day or so. It is not hygroscopic and after half a day, it is perfectly dry and can be transferred to a vial. It is really remarkable how much solid material is obtained from the starting amount of copper(II) oxide.

The dark purple/blue solid is the complex bis(ethylenediamine) copper(II) perchlorate.
![]()
Heating of bis(ethylenediamine) copper(II) perchlorate
The perchlorate salt of the copper(II) complex of ethylenediamine is more sensitive to heat than the nickel-complex and it also seems to decompose somewhat more energetically. The color of the flame is bright blue. Below follow two explosions of a small amount of the solid. The left picture was made in a darkened room, the right picture was made in a room with diffuse daylight (not too strong, overcast weather). These picture were made with a camera in a setting, in which 25 frames per second are made.


A high speed recording was made as well, at 100 frames per second. Below follow two frames, showing the explosion of a small amount (only appr. 2×2×1 mm³). The flash of blue light only lasts for a short time. Only 10 ms later, there is nothing more left than some glowing smoke.


![]()
Reaction of complex with acids
When a small quantity of the solid complex is added to water, then it slowly dissolves. Such a solution has a very intense color. Even a few tiny crystals give a deep blue/purple color to the water. The following picture nicely demonstrates the strength of the color of the complex.

A very dilute solution of the complex was prepared and to that solution, a few drops of moderately diluted acid were added (any acid will do, e.g. hydrochloric acid or sulphuric acid) and the test tube was carefully swirled. This resulted in destruction of the complex in the lower part of the test tube, while near the surface nearly all of the complex still exists. The pale blue color in the lower part of the test tube is due to plain aqueous copper(II) ions, such as present in solutions of copper sulfate.
![]()
Discussion of results
![]() Coordination of ethylenediamine is the same for copper(II) as for nickel(II).
Ethylenediamine is a bidentate ligand in this case. The two nitrogen atoms
coordinate to the central copper atom:
Coordination of ethylenediamine is the same for copper(II) as for nickel(II).
Ethylenediamine is a bidentate ligand in this case. The two nitrogen atoms
coordinate to the central copper atom:
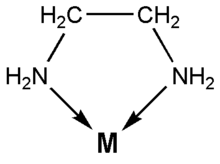
Here M is the copper(II) ion, Cu2+.
![]() The perchlorate salt of the bis(ethylenediamine)
copper complex is soluble quite well. Despite its high solubility, it is easily isolated in solid form. The
dark purple/blue compound
can be written as
The perchlorate salt of the bis(ethylenediamine)
copper complex is soluble quite well. Despite its high solubility, it is easily isolated in solid form. The
dark purple/blue compound
can be written as
[Cu(en)2](ClO4)2
The oxygen balance of this complex salt is not perfect, but it is closer to perfect than the oxygen balance of the nickel complex (which has three ethylenediamine molecules for each 2 perchlorate ions). This explains why the copper complex is more energetic than the nickel complex.
![]() Ethylenediamine is a fairly strong base and is easily protonated by acids, even
if the ligand is coordinated to a metal ion:
Ethylenediamine is a fairly strong base and is easily protonated by acids, even
if the ligand is coordinated to a metal ion:
NH2CH2CH2NH2 + 2H+ → +NH3CH2CH2NH3+
Both amine-groups are protonated by acid and the free electron pair on each of the nitrogen atoms then coordinates to the H+-ion instead of the metal-ion. The ligand then loses contact with the metal ion and the metal ion is coordinated by water again.
back to page about nickel complex