


Synthesis of KBrO3
This page describes how the interesting chemical potassium bromate can be synthesized from potassium bromide. Potassium bromide is easy to obtain. If one has access to potassium dichromate or sodium dichromate, then the synthesis is made more efficient.
Fairly pure potassium bromate can be made easily with carbon electrodes. The potassium bromate can be made completely free of bromide very easily. Getting rid completely of all carbon impurities is harder, but for virtually all practical home chemistry experiments, the product still is perfectly suitable. It is not 100% white, but very pale grey or green/grey.
A perfectly, snow-white product, again completely free of bromide, can easily be obtained when a platinum anode is used during the electrolysis.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium bromide
-
potassium dichromate or sodium dichromate
![]() Required
equipment:
Required
equipment:
-
graphite rods or platinum wire
-
glass tubing
-
power supply, 12 ... 15 V, capable of providing a few A of current, in non-stop operation.
![]() Safety:
Safety:
- Potassium bromate probably is a human carcinogen. There are some doubts on its real toxicity, but it is better to be safe than sorrow.
- Dichromates are toxic and also most likely is a carcinogen.
- During this experiment, quite some hydrogen gas is produced. Do this in a ventilated room, just to be sure that the concentration cannot become high enough to allow an explosion.
![]() Disposal:
Disposal:
- Any waste from this experiment can be flushed down the drain. The amount of dichromate, used in this experiment is really low, it is just a few tens of mg. All other material is either non-toxic, or quickly broken down.
![]()
The general setup
![]() Prepare
a solution of potassium bromide in some distilled water. Prepare a very
concentrated solution by adding more and more KBr, until it does not dissolve
anymore. After that, just add a small amount of water, such that the final
amount of KBr dissolves.
Prepare
a solution of potassium bromide in some distilled water. Prepare a very
concentrated solution by adding more and more KBr, until it does not dissolve
anymore. After that, just add a small amount of water, such that the final
amount of KBr dissolves.
![]() Add a
tiny pinch of potassium dichromate to the solution. It is best to dissolve the
dichromate in 0.5 ml of warm water in a separate test tube and add this to the
solution of KBr. Adding the potassium dichromate directly can be done, but then
it takes a lot of time before it is dissolved. Mix the solution very well. The
amount of potassium dichromate is not critical. The solution must be yellow.
Add a
tiny pinch of potassium dichromate to the solution. It is best to dissolve the
dichromate in 0.5 ml of warm water in a separate test tube and add this to the
solution of KBr. Adding the potassium dichromate directly can be done, but then
it takes a lot of time before it is dissolved. Mix the solution very well. The
amount of potassium dichromate is not critical. The solution must be yellow.
The resulting solution looks as follows:

This is approximately 40 ml of solution, containing approximately 15 grams of KBr and a few tens of mg of K2Cr2O7.
If you do not have any dichromate, then just dissolve some potassium bromide and use the solution as such. Having no dichromate in the solution, however, makes the process less efficient. At a certain point, bromine and bromate are reduced back to bromide, when no dichromate is in the solution.
One could make some chromate by adding potteries green chromium oxide to some sodium hydroxide and some potassium nitrate. Melting this mix results in formation of yellow chromate. A small amount of this yellow material can be dissolved in 30 ml of water, and then carefully hydrochloric acid is dripped in, until the pH is around 7. In this solution one then dissolves the KBr. The small amount of chloride and possibly nitrate, introduced in this way, is not of any concern.
![]() Use a
screw cap for the bottle which holds the solution, with two holes in it, through
which the electrodes can be inserted in the liquid. It is important to have the
bottle/beaker covered with a cap, because of a lot of spraying of fine droplets during the
electrolysis. This is due to the vigorous bubbling of hydrogen out of solution. There should be some space left between the electrodes and the
rims of the holes, because during the electrolysis, hydrogen gas is formed, and
that gas of course must be able to leave the vessel without introducing high
pressure.
Use a
screw cap for the bottle which holds the solution, with two holes in it, through
which the electrodes can be inserted in the liquid. It is important to have the
bottle/beaker covered with a cap, because of a lot of spraying of fine droplets during the
electrolysis. This is due to the vigorous bubbling of hydrogen out of solution. There should be some space left between the electrodes and the
rims of the holes, because during the electrolysis, hydrogen gas is formed, and
that gas of course must be able to leave the vessel without introducing high
pressure.

![]() Build a
circuit, according to the diagram below. Use a resistor, such that the current
is somewhere between 1.5 and 2.5 A. For a 12 V power supply, a good value is
somewhere around 4 Ω. The resistor can become VERY hot, use a big resistor, or
use a parallel and/or series circuit of multiple resistors. Some suggestions for
the power supply and resistors can be found
here. Any other power supply,
which can deliver a constant voltage of 12 to 15 volts is suitable, provided a
resistor or network of resistors is used. Do not connect a 12 to 15 volts power
supply to the cell directly.
Build a
circuit, according to the diagram below. Use a resistor, such that the current
is somewhere between 1.5 and 2.5 A. For a 12 V power supply, a good value is
somewhere around 4 Ω. The resistor can become VERY hot, use a big resistor, or
use a parallel and/or series circuit of multiple resistors. Some suggestions for
the power supply and resistors can be found
here. Any other power supply,
which can deliver a constant voltage of 12 to 15 volts is suitable, provided a
resistor or network of resistors is used. Do not connect a 12 to 15 volts power
supply to the cell directly.
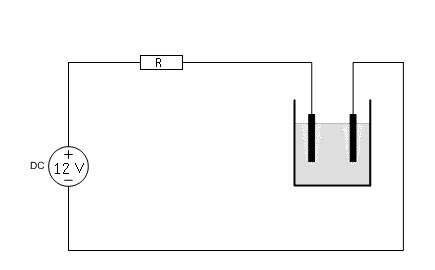
Two versions of the circuit were built. One used two graphite electrodes, one for the anode and one for the cathode. The other circuit used a platinum anode, made from two platinum wires and a titanium cathode. More details on these are given in the sections below.
![]()
The choice of electrodes
It really does matter what kind of electrodes are used, especially the choice of anode material is critical. The anode material must be really oxidation resistant. The best choice is platinum, but not everyone has a platinum anode. An acceptable alternative is graphite. Graphite rods can be purchased at some welding shops, but they can also be taken from the good old zinc/carbon batteries. They cannot be taken from more modern batteries, like alkaline or lithium cells.
The choice of the cathode material is less critical. As long as the cell is operational, the cathode is not attacked at all, but as soon as the power supply is switched off, the cathode will be oxidized by the hot and strongly oxidizing solution. For this reason it is best to use a graphite cathode, or a cathode of a fairly corrosion resistant metal, such as stainless steel, titanium, or nickel. Copper is less suitable, it quickly dissolves when the power supply is not switched on, and this can lead to copper contamination.
Platinum electrodes
In one run of the synthesis, platinum electrodes were used. These platinum electrodes are home-made from platinum wire, purchased from an eBay seller (uGems). The wire is soldered to a thick copper wire, and the platinum is melted inside a thin glass tube. Two pieces of wire were available (25 cm of 0.3 mm wire, pure platinum, and 12.5 cm of 0.4 mm of a 95% Pt/5% Ir alloy). Both wires were placed in parallel in the solution, making a bigger anode. These electrodes look as follows:
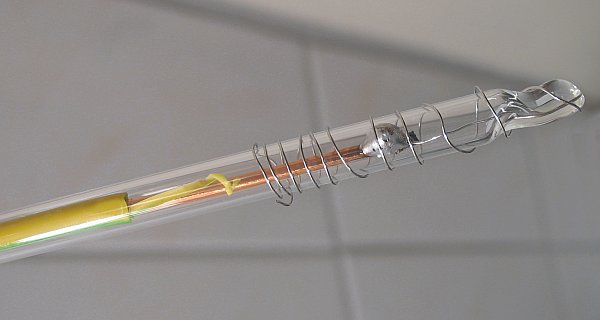

Melting the glass was done with a small butane torch. These glass sticks, with the platinum wire around them are immersed in the liquid. The glass protects the copper wire from the corrosive liquid.
Graphite electrodes
The graphite electrodes were taken from a 6V zinc/carbon battery, type 4R25. These batteries are cheap, and they contain 4 nice thick rods. It is, however, quite a messy and dirty job to take out these rods.

The picture below shows all electrodes, the two platinum wires, both used as anode in one experiment, and one graphite rod from a 4R25 battery.

The holes in the red screw cap of the beaker are such, that all types of electrodes can easily be inserted into the liquid, through the holes.
Titanium electrode
In the synthesis, using both platinum electrodes as anode, a titanium cathode is used. In this synthesis, both platinum anodes are inserted through the two holes in the screw cap, and the cathode was bent in a circular way, such that it could be pushed to the bottom of the beaker. It has a thickness of 1.5 mm and as such, it could also go through one of the holes, together with the glass tube of one of the anodes. The anodes were kept a little above the thick titanium cathode, assuring that no short circuit is built in the liquid, and assuring that hydroxide, produced at the cathode moves along the anode.

![]()
Synthesis with graphite anode and graphite cathode
A current of approximately 2 A was running through the cell for almost 6 hours. During this electrolysis, the cell became quite warm. It was so warm, that it was just bearable to keep it in bare hands. Estimated temperature is somewhere between 50 and 60 °C.
 After
two hours or so, the first amount of solid potassium bromate can be observed as dirty
off-white crystals, settling at the bottom. After 6 hours, the power supply was
switched off, the electrodes were taken out of the solution and the solution
was allowed to cool down.
After this time, the solution is very dark green, filled with lots of solid
particles from the graphite anode. The anode erodes somewhat, the cathode is not
affected at all. The picture shows the result after 5 to 6 hours of electrolysis
with the graphite electrodes.
After
two hours or so, the first amount of solid potassium bromate can be observed as dirty
off-white crystals, settling at the bottom. After 6 hours, the power supply was
switched off, the electrodes were taken out of the solution and the solution
was allowed to cool down.
After this time, the solution is very dark green, filled with lots of solid
particles from the graphite anode. The anode erodes somewhat, the cathode is not
affected at all. The picture shows the result after 5 to 6 hours of electrolysis
with the graphite electrodes.
At the bottom, the dirty off-white raw and impure KBrO3 can be observed. Above the solid there is a dark liquid, with a green color, and black solid particles.
Allow this liquid to cool down in a fridge, such that it becomes ice-cold (but it may not freeze). If this is done, then almost 100% of the KBrO3 crystallizes at the bottom. Also some solid may form at the surface of the liquid.
Getting rid of the carbon completely is very difficult. One first has to stir the liquid vigorously with a plastic or glass stirrer (don't use metal, it contaminates the liquid and it is corroded). The small carbon particles remain floating in the liquid for quite some time, while the crystals of potassium bromate quickly settle at the bottom again. When almost all crystals of the potassium bromate have settled again, quickly decant the dirty green liquid. This removes most carbon. Now take some ice-cold water again (just above 0 °C) and add this to the dirty crystals of potassium bromate. Then stir again and quickly decant in order to minimize the loss of potassium bromate, due to dissolving in the rinsing water. The rinse water looks as follows, and already is much better.

As the picture shows, there still is some carbon, and also a yellow/green solution. The solid is not purely white, but somewhat dirty-white. Quickly decant the ice cold water again.
Now, after this rinse, one could use another rinse, in a very small amount of ice cold water, which just covers the solid. This second rinse again may remove some carbon and some green/yellow material.
At this stage, one has raw green/grey potassium bromate, which, when dried, is suitable as a solid oxidizer and reacts very energetically with many reductors like sulphur, fine metal powders and red phosphorus.
Refining/recrystallizing the raw potassium bromate
This stage only is required if the potassium bromate is used for other purposes than as a solid oxidizer in (small) pyro experiments. The raw potassium bromate contains some potassium bromide as well, and this potassium bromide can be removed very well, due to the large difference of the solubility of these chemicals in ice cold water. Any remains of dichromate also can easily be removed. Removal of ultrafine carbon particles is much harder and getting totally rid of that impurity probably will take several carefully conducted recrystallizations, but of course that also introduces a significant loss.
The refining must be done as follows:
![]() Put the raw (and still wet) potassium bromate in a
heat-resistant glass beaker and add the same volume of distilled water. Then heat the
beaker, until the solution just starts to boil. Then stir and see how much
potassium bromate dissolves. Carefully add small amounts of distilled water,
while keeping the solution hot and stirring occasionally. Do this, until all
potassium bromate has dissolved. Add 1 or 2 ml of extra water. Now keep the
solution hot for several minutes, but without boiling. Any solid matter now
settles at the bottom, a light and clear green/yellow solution is obtained.
Carefully decant this solution in another pre-heated clean beaker, assuring that
no solid particles come into the other beaker. This can be somewhat tricky.
Using a filter is not an option here, because the cold filter will cause
immediate crystallization of potassium bromate and this will clog the filter and
will lead to severe losses. A small part of the liquid cannot be decanted into
the other beaker and this should be accepted as loss. It still can be used for
the more raw experiments.
Put the raw (and still wet) potassium bromate in a
heat-resistant glass beaker and add the same volume of distilled water. Then heat the
beaker, until the solution just starts to boil. Then stir and see how much
potassium bromate dissolves. Carefully add small amounts of distilled water,
while keeping the solution hot and stirring occasionally. Do this, until all
potassium bromate has dissolved. Add 1 or 2 ml of extra water. Now keep the
solution hot for several minutes, but without boiling. Any solid matter now
settles at the bottom, a light and clear green/yellow solution is obtained.
Carefully decant this solution in another pre-heated clean beaker, assuring that
no solid particles come into the other beaker. This can be somewhat tricky.
Using a filter is not an option here, because the cold filter will cause
immediate crystallization of potassium bromate and this will clog the filter and
will lead to severe losses. A small part of the liquid cannot be decanted into
the other beaker and this should be accepted as loss. It still can be used for
the more raw experiments.
![]() Let the clear liquid cool down. First at room temperature,
and finally in a fridge, such that it becomes ice cold. Almost all potassium
bromate settles at the bottom as a crystalline solid, which is almost white now.
There still is some green/grey color, but it is very faint and for all
home-experiments, this is good enough.
Let the clear liquid cool down. First at room temperature,
and finally in a fridge, such that it becomes ice cold. Almost all potassium
bromate settles at the bottom as a crystalline solid, which is almost white now.
There still is some green/grey color, but it is very faint and for all
home-experiments, this is good enough.
![]() While the liquid still is ice cold, decant the liquid and try
to remove as much as possible of the liquid from the crystals. The cold solution
can be filtered, keeping the crystals, but decanting also works quite well. The
ice-cold liquid can be thrown away, it hardly contains any potassium bromate.
Try to get rid of as much as possible of the solution, sticking to the solid. A
nice method is the use of a dry and clean paper tissue (e.g. Kleenex), which is
wound into a small cylinder of tissue and the end of this cylinder is pressed
gently against the wet potassium bromate. Due to capillary forces, most of the
water is slowly sucked away from the potassium bromate into the paper tissue.
While the liquid still is ice cold, decant the liquid and try
to remove as much as possible of the liquid from the crystals. The cold solution
can be filtered, keeping the crystals, but decanting also works quite well. The
ice-cold liquid can be thrown away, it hardly contains any potassium bromate.
Try to get rid of as much as possible of the solution, sticking to the solid. A
nice method is the use of a dry and clean paper tissue (e.g. Kleenex), which is
wound into a small cylinder of tissue and the end of this cylinder is pressed
gently against the wet potassium bromate. Due to capillary forces, most of the
water is slowly sucked away from the potassium bromate into the paper tissue.
![]() Now rinse the still wet crystals two times with a small
amount of ice cold water. The volume of water must be 2 to 3 times the volume of
the crystal mass, not more. After each rinse, again try to get rid of as much as
possible of the water.
Now rinse the still wet crystals two times with a small
amount of ice cold water. The volume of water must be 2 to 3 times the volume of
the crystal mass, not more. After each rinse, again try to get rid of as much as
possible of the water.
![]() Finally let the crystals dry in contact with air, on a warm
place. Above a warm heat radiator is a very good place. Within one day, perfectly dry
crystals are obtained. Potassium bromate is not hygroscopic at all.
Finally let the crystals dry in contact with air, on a warm
place. Above a warm heat radiator is a very good place. Within one day, perfectly dry
crystals are obtained. Potassium bromate is not hygroscopic at all.
![]()
Synthesis with platinum anodes and a titanium cathode
Again, a current of approximately 2 A was run through the cell for 5 to 6 hours. After this period, the liquid has a nice layer of potassium bromate at the bottom, and above that, a clear yellow liquid is obtained. The result is much cleaner than with a graphite anode. The liquid is still yellow, due to the dichromate. It seems to be a little bit more yellow than it was before. Probably this is due to slight conversion of chromium (VI) to chromium (III), resulting in a shift towards green. Also, slight loss of bromine through evaporation from the liquid may cause this effect. Loss of bromine results in slight excess of hydroxide, making the color shift from orange/yellow to lemon yellow.
The picture below shows the result after the electrolysis. The solid material again can be seen on the bottom. It is clean-white. It is remarkable how white this material is. Apparently, the dichromate is not incorporated in the crystal lattice when the material crystallizes from the solution.

Cleaning of the potassium bromate from this cell was done in the same way as with the graphite cells. The cleaning now is easier, because there are no solid particles of carbon, but the process is very similar: decanting, rinsing and the use of very cold water. Again, the solid was recrystallized to make it really pure and to get rid of potassium bromide in the solid.
![]()
The result of both processes
The final result is very useful, in both cases. The material, obtained from the carbon-carbon electrolysis, however, is not totally white. The result of the platinum-titanium electrolysis is purely white.

The picture nicely shows the difference between the two samples. The material from the carbon-carbon electrolysis has a faint green color, which is remarkably difficult to remove. Even another recrystallization from distilled water did not remove the green color. Probably this green color is due to ultrafine carbon particles, with adsorbed chromium (III) and/or dichromate. When no dichromate is used, then the potassium bromate tends to become grey, due to very small carbon particles. When dichromate is used, then these black particles may contain some dichromate or chromium (III) as well and this could cause a green/grey color. These ultrafile carbon particles are very hard to remove, they cannot be filtered, because of their extremely small size and they tend to be included in the recrystallized KBrO3.
In chemical experiments, however, the green/grey material performs equally well as the white material. The green/grey material was used with success in a Belousov-Zhabotinsky reaction. It also was used for making Ba(BrO3)2.
Both batches of potassium bromate were tested for the presence of bromide, by adding a spatula full of the solid to dilute sulphuric acid. This also was compared with a batch which was not recrystallized.
The recrystallized material, when added to 2 M H2SO4 hardly shows any yellow color, when it dissolves. When no refining/recrystallization step is performed, then the dilute acid becomes clearly yellow/orange on addition of some potassium bromate. Comparison with another bromine solution shows that without recrystallization and subsequent rinsing certainly 1 or 2% of the solid is KBr. So, the recrystallization/rinse step really is necessary to get rid of bromide. On the other hand, this test also shows that the recrystallization/rinse step is very effective at removing bromide.
Possible optimizations for improving the yield
The yield of the syntheses is not yet optimal. In order to obtain better yields, it is necessary to run the cells for a longer time, and probably by taking even more care in the refining process, the yield also can be increased. Using higher concentrations of potassium dichromate also may increase the yield, but it also adds more impurity. Especially in the case of the use of platinum anodes, the yellow liquid could be reused, by dissolving some new potassium bromide in it, and using that for further production of potassium bromate. Using that technique, the yield per batch is not increased, but the remains of an old batch then can be used as a start for a new batch and much less KBr is needed to make a new batch.
![]()
The underlying principle of this synthesis
The net reaction in the electrolysis cell is the following:
Br– + 3H2O → BrO3– + 3H2
The mechanism, leading to this net reaction is as follows:
- At anode, which absorbs electrons: 2Br– – 2e → Br2
- At cathode, which produces electrons: 2H2O + 2e → 2OH– + H2
Due to mixing of the liquid, the bromine, formed at the anode, reacts with the hydroxide ions, formed at the cathode:
Br2 + 2OH– → BrO– + Br– + H2O
The hypobromite (BrO–) quickly disproportionates in the warm liquid:
3BrO– → BrO3– + 2Br–
The bromide, formed again in this reaction, in turn can be oxidized at the anode again, forming bromine. In this way, finally all bromide ions are used up and all is converted to bromate ions.