


Sulphur
Sulphur exists in at least three allotropes, which all are yellow in the solid state. There are two crystalline allotropes and a plastic-like allotrope (and probably some more, which are not covered here). The two crystalline allotropes are rhombic sulphur and monoclinic sulphur. Rhombic sulphur is the stable form at room temperature. When rhombic sulphur is heated carefully at approximately 100 °C (e.g. by putting it in boiling water), then it slowly is converted to monoclinic sulphur. Below, pictures are shown of ideal crystals of rhombic sulphur and monoclinic sulphur.
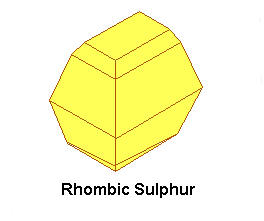
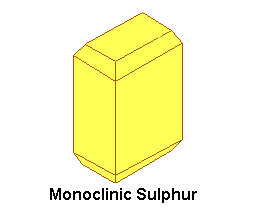
Rhombic sulphur and monoclinic sulphur both consist of S8 molecules, but ordered in a different way in the two different types of crystal. When sulphur is heated, then it melts at a certain temperature, forming a mobile yellow liquid. When the sulphur is heated to a higher temperature, then the liquid becomes more and more viscous and it also darkens. This is due to breakdown of the S8 molecules and formation of long chains of S-atoms. When this viscous liquid is cooled down very quickly (e.g. by pouring it in a beaker of cold water), then the plastic allotrope is formed. This is an amorphous solid, which slowly reverts to the rhombic form on standing.
Ordinary sulphur is rhombic sulphur. It can be purchased in three forms:
- Flowers of sulphur is the sublimed substance from purification by distillation. It contains a little oxygen, and possibly a tiny amount of sulphuric acid created by slow oxidation, but these are the only impurities. Its colloidal form makes the powder very active, and this is the only hazard involved in its use.
- Roll sulphur is cast rhombic sulphur, usually not as pure as flowers.
- Sulphur flour is ground rhombic sulphur, and the powder size is larger than in flowers of sulphur.
 Flowers of sulphur,
as shown in the picture, should be used for chemical purposes,
as it is the purest form. It, however, should never be used as a part of any
pyrotechnic mixture. The experiments on this site all can be performed with
flowers of sulphur. Flowers of sulphur is the usual form of sulphur, sold by
drugstores. It is recommended to have some sulphur in the home lab. It can be
used in many interesting experiments, both in aqueous solutions and in
dry-chemical experiments.
Flowers of sulphur,
as shown in the picture, should be used for chemical purposes,
as it is the purest form. It, however, should never be used as a part of any
pyrotechnic mixture. The experiments on this site all can be performed with
flowers of sulphur. Flowers of sulphur is the usual form of sulphur, sold by
drugstores. It is recommended to have some sulphur in the home lab. It can be
used in many interesting experiments, both in aqueous solutions and in
dry-chemical experiments.
![]() Dry-chemical
experiments can also be performed with flowers of sulphur, but pyrotechnic
mixtures, containing flowers of sulphur should be used immediately after
preparation. They should never be stored! The possible acid in the
flowers of sulphur may slowly sensitize such mixtures, which may in the worst
case lead to self-ignition.
Dry-chemical
experiments can also be performed with flowers of sulphur, but pyrotechnic
mixtures, containing flowers of sulphur should be used immediately after
preparation. They should never be stored! The possible acid in the
flowers of sulphur may slowly sensitize such mixtures, which may in the worst
case lead to self-ignition.
Sulphur burns in air with a pale blue flame. While it is burning, it mainly is converted to sulphur dioxide. A small amount is converted to sulphur trioxide, which is the thin white smoke, which can be observed when sulphur is burned.
![]()
There are many compounds of sulphur, which are interesting for the home lab. Probably sulphur is the element which has the largest number of inorganic compounds, which are available to the general public and which are interesting to be used in a home lab. Before going into detail some generic information on sulphur compounds is given first.
Sulphur exists in many oxidation states in its compounds. Most important are the oxidation states -2, 0, +2, +3, +4 and +6, but oxidation state +5 also is present in stable salts. Some compounds seem to have other oxidation states, but usually these are mixed-oxidation state compounds. Sulphur has the property that it easily forms oxo-anions with multiple sulphur atoms, which are attached to each other and even can form chains. These different sulphur atoms in most of these anions are not equivalent and hence are assigned different oxidation numbers.
The sulfate ion is an anion, which is part of many inorganic compounds. Many metal compounds are available as the metal sulfates (e.g. copper, iron, manganese, nickel, cobalt, vanadyl). The reason for this is that sulfate is a very inert ion in aqueous solutions. The properties of these salts then are mainly determined by the cation. Other non-metal examples of such salts are ammonium sulfate, diethyleneamine sulfate and hydroxyl amine sulfate. These latter three are covered in the section on nitrogen, because their main properties are determined by the anion.
Below follows a list of compounds, which have properties, mainly determined by their sulphur-containing anion. Many of these should be present in a well-equipped home lab, because they have interesting properties.
- Oxidation state +6, sodium sulfate, Na2SO4, white solid
- Oxidation state +6, sodium bisulfate, NaHSO4, white solid
- Oxidation state +6, sulphuric acid, H2SO4, colorless liquid
- Oxidation state +6, sodium persulfate, Na2S2O8, white solid
- Oxidation state +5, sodium dithionate, Na2S2O6, white solid
- Oxidation state +4, sodium sulfite, Na2SO3, white solid
- Oxidation state +4, sodium metabisulfite, Na2S2O5, white solid
- Oxidation state +6 and -2, sodium thiosulfate, Na2S2O3, white solid
- Oxidation state +3, sodium dithionite, Na2S2O4, white solid
- Oxidation state -2, sodium sulfide, Na2S, white or yellow solid
- Oxidation state -2 and 0, potassium polysulfide, also known as liver of sulphur, K2Sx, brown solid
- Oxidation state -2, thiourea, (NH2)2CS, white solid
![]() Sodium sulfate is the most common sulfate compound, but also the least
interesting. Both the anion and the cation are unreactive. However, sometimes
this is beneficial. Dissolving sodium sulfate in water, allows one to make a
solution with some content, which does not react easily. This is exploited
sometimes because of osmotic effects and in certain electrochemical experiments.
In photography, sodium sulfate solutions are used to replace chemically active
solutions in the print-paper by sodium sulfate solution. The latter then can be
rinsed out slowly and the active solution cannot act upon the print anymore.
Sodium sulfate is available as anhydrous salt, being a white powder, or as its
decahydrate, being a transparent crystalline solid. The decahydrate of sodium
sulfate is called Glauber's salt. Either one of these can be purchased in better
equipped drugstores. Sodium sulfate makes large crystals remarkably easily and
for this purpose it can be quite interesting to experiment with sodium sulfate.
Sodium sulfate is the most common sulfate compound, but also the least
interesting. Both the anion and the cation are unreactive. However, sometimes
this is beneficial. Dissolving sodium sulfate in water, allows one to make a
solution with some content, which does not react easily. This is exploited
sometimes because of osmotic effects and in certain electrochemical experiments.
In photography, sodium sulfate solutions are used to replace chemically active
solutions in the print-paper by sodium sulfate solution. The latter then can be
rinsed out slowly and the active solution cannot act upon the print anymore.
Sodium sulfate is available as anhydrous salt, being a white powder, or as its
decahydrate, being a transparent crystalline solid. The decahydrate of sodium
sulfate is called Glauber's salt. Either one of these can be purchased in better
equipped drugstores. Sodium sulfate makes large crystals remarkably easily and
for this purpose it can be quite interesting to experiment with sodium sulfate.
![]() Sodium bisulfate usually is applied because of the relatively strong acidity of
its solutions. It sometimes can be used as a safer and easier to handle
alternative to sulphuric acid, when the presence of the sodium ions does not
interfere. Sodium bisulfate can be ordered from photography raw chemical
suppliers and it can also be purchased as pH-minus for swimming pools. It comes
in the form of white pearls, which can be dissolved in water easily.
Sodium bisulfate usually is applied because of the relatively strong acidity of
its solutions. It sometimes can be used as a safer and easier to handle
alternative to sulphuric acid, when the presence of the sodium ions does not
interfere. Sodium bisulfate can be ordered from photography raw chemical
suppliers and it can also be purchased as pH-minus for swimming pools. It comes
in the form of white pearls, which can be dissolved in water easily.
![]() Sulphuric acid is a colorless viscous liquid, which is miscible with water in
all proportions.
Sulphuric acid is a colorless viscous liquid, which is miscible with water in
all proportions. ![]()
![]() It is an extremely
corrosive liquid, which chars skin, paper, tissue, sugar and many other organic
compounds. When working with the concentrated acid, never allow it to come in
contact with the skin and certainly not with the eyes!
It is an extremely
corrosive liquid, which chars skin, paper, tissue, sugar and many other organic
compounds. When working with the concentrated acid, never allow it to come in
contact with the skin and certainly not with the eyes!
![]() When the
concentrated acid is mixed with water, then a lot of heat is produced.
Water should never be added to the acid, but acid must always be added to water,
slowly and constantly stirring. When water is poured on acid, then the water may
start boiling at once, swirling hot water and acid out of the container!
When the
concentrated acid is mixed with water, then a lot of heat is produced.
Water should never be added to the acid, but acid must always be added to water,
slowly and constantly stirring. When water is poured on acid, then the water may
start boiling at once, swirling hot water and acid out of the container!
Solutions of sulphuric acid in water are strongly acidic. In spite of the corrosiveness of the concentrated acid, it is suggested to have some sulphuric acid for the home lab. This acid is strong, non-reducing and even at fairly high concentrations also non-oxidizing. Besides that, the sulfate ion also does not coordinate to many compounds at low pH. All these properties make dilute sulphuric acid very attractive for many experiments, where one only wants strong acidity without other interfering effects. If the concentrated acid is purchased, then it might be wise to prepare a larger amount of dilute acid (e.g. from 100 ml of acid and 1 liter of water) at once, such that the dangerous act of diluting the acid only needs to be done once.
Sulphuric acid, although very corrosive, is not poisonous. The diluted acid is much less corrosive. Waste, containing dilute sulphuric acid can be flushed down the drain with lots of water without bad effect for the environment. In the environment the acid is quickly neutralized to sulfate, which is non-toxic.
![]() Sodium persulfate probably is the strongest oxidizer, available to the public. A
more thorough description of this compound is given in the
section on oxygen. Alternatives for sodium
persulfate might be ammonium persulfate or potassium persulfate. The latter
dissolves with more difficulty, but it still is sufficiently soluble in water to
allow interesting redox-experiments to be performed. Sodium persulfate,
potassium persulfate and ammonium persulfate all are white crystalline solids.
Ammonium persulfate stores not as well as the other two, it tends to become
humid, and then it looses oxygen, itself being converted to ammonium bisulfate.
Sodium persulfate probably is the strongest oxidizer, available to the public. A
more thorough description of this compound is given in the
section on oxygen. Alternatives for sodium
persulfate might be ammonium persulfate or potassium persulfate. The latter
dissolves with more difficulty, but it still is sufficiently soluble in water to
allow interesting redox-experiments to be performed. Sodium persulfate,
potassium persulfate and ammonium persulfate all are white crystalline solids.
Ammonium persulfate stores not as well as the other two, it tends to become
humid, and then it looses oxygen, itself being converted to ammonium bisulfate.
![]() Sodium dithionate is an intermediate between sodium sulfate and sodium sulfite.
Despite the unusual oxidation state of sulphur in this compound, it is
remarkably stable. Dithionate ion is resistant, both to oxidation and reduction.
The acid also is quite stable in aqueous solution, but it cannot be isolated as
pure acid. If that is attempted, then it decomposes to sulphuric acid and all
kinds of oxo-sulphur compounds with sulphur at lower oxidation states.
Sodium dithionate is an intermediate between sodium sulfate and sodium sulfite.
Despite the unusual oxidation state of sulphur in this compound, it is
remarkably stable. Dithionate ion is resistant, both to oxidation and reduction.
The acid also is quite stable in aqueous solution, but it cannot be isolated as
pure acid. If that is attempted, then it decomposes to sulphuric acid and all
kinds of oxo-sulphur compounds with sulphur at lower oxidation states.
![]() Sodium sulfite is available in two forms, either as the heptahydrate or as
anhydrous salt. The latter is preferred, because it keeps well. The heptahydrate
is efflorescent and while loosing crystal water, it is sensitive to
air-oxidation, the solid being converted to sodium sulfate. The hydrated sodium
sulfite is a transparent crystalline solid, consisting of large hexagonal
crystals (some crystals almost have a diameter of 10 mm). The anhydrous sodium
sulfite is a fine crystalline white powder.
Sodium sulfite is available in two forms, either as the heptahydrate or as
anhydrous salt. The latter is preferred, because it keeps well. The heptahydrate
is efflorescent and while loosing crystal water, it is sensitive to
air-oxidation, the solid being converted to sodium sulfate. The hydrated sodium
sulfite is a transparent crystalline solid, consisting of large hexagonal
crystals (some crystals almost have a diameter of 10 mm). The anhydrous sodium
sulfite is a fine crystalline white powder.
Sulfite is a facile reductor, especially in acidic environments, where it is converted to sulphur dioxide. It reduces compounds like permanganates and dichromates quickly and completely. This is a strong difference with organic reductors, which act slowly and often incompletely.
Sodium sulfite can be purchased at some better equipped drugstores. It also can be ordered from photography raw chemical suppliers.
Sodium sulfite is not particularly toxic or corrosive. One must, however, keep in mind that the sulphur dioxide, formed on acidification, is very pungent. It has a sharp odor and at higher concentration it is toxic. It, however, does not have adverse long term effects and has a good warning level.
![]() Sodium metabisulfite is a white crystalline solid, resembling sodium sulfite in
many respects. A solution of sodium metabisulfite in water is completely
equivalent to an equimolar solution of sodium sulfite, with the same molar
concentration of sulphur dioxide. So, sodium metabisulfite can be regarded as a
somewhat more acidic variation of sodium sulfite. When one has sodium sulfite,
then there is less need for having sodium metabisulfite. For many experiments
these chemicals can be exchanged with each other. Sodium metabisulfite is
available from photography raw chemical suppliers. An equally well working
substitute, potassium metabisulfite, is available at brewery and wine-making
shops. Many drugstores also have at least one of the metabisulfites.
Sodium metabisulfite is a white crystalline solid, resembling sodium sulfite in
many respects. A solution of sodium metabisulfite in water is completely
equivalent to an equimolar solution of sodium sulfite, with the same molar
concentration of sulphur dioxide. So, sodium metabisulfite can be regarded as a
somewhat more acidic variation of sodium sulfite. When one has sodium sulfite,
then there is less need for having sodium metabisulfite. For many experiments
these chemicals can be exchanged with each other. Sodium metabisulfite is
available from photography raw chemical suppliers. An equally well working
substitute, potassium metabisulfite, is available at brewery and wine-making
shops. Many drugstores also have at least one of the metabisulfites.
![]() Sodium thiosulfate is a solid, consisting of fairly large crystals. It usually
comes as the pentahydrate, although the anhydrous form also sometimes is found.
The hydrated solid already 'melts' at a temperature of approximately 40 degrees.
It is not real melting, but loosing water of crystallization and dissolving in
this lost water.
Sodium thiosulfate is a solid, consisting of fairly large crystals. It usually
comes as the pentahydrate, although the anhydrous form also sometimes is found.
The hydrated solid already 'melts' at a temperature of approximately 40 degrees.
It is not real melting, but loosing water of crystallization and dissolving in
this lost water.
Sodium thiosulfate is a mild reducing agent and a strong coordinating agent. Many metal cations form complexes with thiosulfate. Photographic fixers are based on this property of thiosulfate. Insoluble compounds, like unexposed and not developed AgCl and AgBr in the developed print are broken apart by the thiosulfate, present in photo fixers. The silver ion goes into solution as a thiosulfato complex and the chloride or bromide ion goes into solution as a plain aquated ion. In the riddles section on this site, an experiment is given, where thiosulfate forms a complex with copper ions.
On acidification, the thiosulfate ion forms the weak acid thiosulphuric acid, H2S2O3. This acid is unstable and falls apart, one of the products being sulphur and the other being sulphurous acid, which in turn breaks down to water and sulphur dioxide. There also are many side reactions. Acidified thiosulfate hence contains a complex mixture of many sulphur compounds and elementary sulphur, which is finely divided in the liquid.
Sodium thiosulfate is available at some drugstores, it can also be ordered at photography raw chemical suppliers. In some countries, it may also be sold by photography shops. Sodium thiosulfate keeps indefinitely, if kept dry. This compound should be present in the home lab.
![]() Sodium dithionite is a white powder. This compound has a strong smell of sulphur
dioxide. The dry compound is quite stable. When it becomes wet, it quickly
decomposes. Sodium dithionite is flammable, when it becomes moist, it may even
become pyrophoric!
Sodium dithionite is a white powder. This compound has a strong smell of sulphur
dioxide. The dry compound is quite stable. When it becomes wet, it quickly
decomposes. Sodium dithionite is flammable, when it becomes moist, it may even
become pyrophoric!
A solution of sodium dithionite in water slowly turns yellow. Such a solution produces the smell of sulphur dioxide, but also of other noxious sulphur compounds (it really stinks). After several minutes, free sulphur is deposited. A fresh solution of sodium dithionite is a very strong reductor. It reduces many metal ions to the metallic state (e.g. copper, silver, bismuth).
When an acid is added to a fresh solution of sodium dithionite, then a red compound is formed, which quickly fades. The nature of the red compound is not yet known for sure, but it it thought that this is dithionic acid. After a minute or so, this red compound decomposes and sulphur is precipitated from the liquid.
Sodium dithionite is a nice compound to have in the home lab. It is a strong reductor. Unfortunately it is not a clean reductor. The unused part of this compound gives a mess in the solutions.
![]() Sodium sulfide usually comes in the form of light yellow flakes. This is the
technical grade compound. Analytic grade sodium sulfide is a colorless
crystalline solid, which comes in the form of the nonahydrate. For the home
chemist, the technical grade compound is sufficient. It is available from
photography raw chemical suppliers and it is cheap.
Sodium sulfide usually comes in the form of light yellow flakes. This is the
technical grade compound. Analytic grade sodium sulfide is a colorless
crystalline solid, which comes in the form of the nonahydrate. For the home
chemist, the technical grade compound is sufficient. It is available from
photography raw chemical suppliers and it is cheap.
Solutions of sodium sulfide and to a lesser extent the solid as well, have a
horrible smell of rotten eggs. This is the smell of hydrogen sulfide. As long as
no acid is involved in the experiments, there is no need to worry about the
smell, other than that it is unpleasant. If acids are added, even weak ones like
vinegar, then the concentration of hydrogen sulfide can become so high, that it
becomes a serious health risk.
![]() Be warned: hydrogen
sulfide has a strong odor, but at higher concentration, it tends to kill your
sense of smell. This makes working with sulfides in acidic media dangerous. If
quantities are kept below 100 mg, then one does not run the risk of getting
knocked out by the gas in an average room, but nevertheless, experiments with
sulfides can better be performed outside.
Be warned: hydrogen
sulfide has a strong odor, but at higher concentration, it tends to kill your
sense of smell. This makes working with sulfides in acidic media dangerous. If
quantities are kept below 100 mg, then one does not run the risk of getting
knocked out by the gas in an average room, but nevertheless, experiments with
sulfides can better be performed outside.
From the hobby chemist's point of view, sodium sulfide is an interesting compound. It can be used for interesting gas experiments with small quantities of hydrogen sulfide. It also has an interesting aqueous chemistry. Sulfide is a strong reductor, which is easily oxidized to elementary sulphur. It can be used for making all kinds of precipitates with several metals. It also shows remarkable reactions with elementary sulphur and selenium, but also with some common organic compounds.
![]() Potassium polysulfide is a yellow/brown solid, which dissolves readily in water,
forming a golden yellow solution. Polysulfide solutions can also be prepared by
dissolving sulphur in a solution of a sulfide. When one has access to sodium
sulfide, then there is no specific reason to purchase potassium polysulfide. The
compound, which sometimes is sold as 'liver of sulphur', is quite expensive. It
can be ordered at photography raw chemical suppliers.
Potassium polysulfide is a yellow/brown solid, which dissolves readily in water,
forming a golden yellow solution. Polysulfide solutions can also be prepared by
dissolving sulphur in a solution of a sulfide. When one has access to sodium
sulfide, then there is no specific reason to purchase potassium polysulfide. The
compound, which sometimes is sold as 'liver of sulphur', is quite expensive. It
can be ordered at photography raw chemical suppliers.
![]() Thiourea is a white crystalline solid, which easily can be dissolved in water.
In alkaline aqueous solutions, it hydrolyses and produces small quantities of
free sulfide. For this reason, it sometimes is used as an odorless replacement
for solutions of sodium sulfide. An example of such use in place of plain
sulfide is in photography as a brown/sepia toner. Thiourea is quite interesting.
It forms coordination complexes with several metals. It also is a strong
reductor in acidic environments, in which it easily is converted to formamidine
disulfide.
Thiourea is a white crystalline solid, which easily can be dissolved in water.
In alkaline aqueous solutions, it hydrolyses and produces small quantities of
free sulfide. For this reason, it sometimes is used as an odorless replacement
for solutions of sodium sulfide. An example of such use in place of plain
sulfide is in photography as a brown/sepia toner. Thiourea is quite interesting.
It forms coordination complexes with several metals. It also is a strong
reductor in acidic environments, in which it easily is converted to formamidine
disulfide.
![]() Be careful, when
oxidizing thiourea. Do not carelessly use dilute nitric acid as the proton-donor
in the redox reaction. Also assure than no nitrate ion is in the mixture. The
oxidation product formamidine disulfide forms a very sparingly soluble
nitrate-salt, which auto-ignites on storage! The experiments can be safely
conducted with hydrochloric acid and sulphuric acid.
Be careful, when
oxidizing thiourea. Do not carelessly use dilute nitric acid as the proton-donor
in the redox reaction. Also assure than no nitrate ion is in the mixture. The
oxidation product formamidine disulfide forms a very sparingly soluble
nitrate-salt, which auto-ignites on storage! The experiments can be safely
conducted with hydrochloric acid and sulphuric acid.
![]() Thiourea is a
probable human carcinogen. Be extra careful not to inhale thiourea dust.
Thiourea is a
probable human carcinogen. Be extra careful not to inhale thiourea dust.
![]()
Besides the compounds of sulphur, mentioned above, there are several other compounds of sulphur, which are available for the general public. Some of them are organic compounds. Below follows a short list of other possibly interesting sulphur compounds, without description. For more info, see textbooks and the Internet.
- carbon disulfide, CS2 (toxic, highly flammable)
- dimethyl sulphoxide, often abbreviated as DMSO, (CH3)2SO
- sodium thiocyanate, NaSCN, see section on carbon
- antimony sulfide, Sb2S3
- ferrous sulfide, FeS