


Riddle: Copper (II) and thiosulfate
Copper (II) reacts with thiosulfate in a peculiar way. Probably the reaction between copper (II) and thiosulfate is a combination of redox chemistry and coordination chemistry. The compounds formed in the reaction depend on the concentration of the reactants and the relative total amounts of the reactants. Most peculiar is that the compounds, formed in the reaction between copper (II) and thiosulfate at high concentration cannot be converted to reaction products, formed at low concentration. Dilution of the high concentration reaction products does not result in the products formed at lower initial concentration.
The questions, raised during these experiments are: What is the exact nature of the yellow compounds formed? Is there a real difference between the solid yellow compound and the dissolved yellow compound?
![]()
![]() Required
chemicals:
Required
chemicals:
-
copper sulfate pentahydrate
- sodium thiosulfate pentahydrate
![]() Required
equipment:
Required
equipment:
-
test tubes
![]() Safety:
Safety:
- Copper sulfate is moderately toxic.
![]() Disposal:
Disposal:
-
Copper compounds are moderately toxic for the environment. It is best to bring copper waste to a chemical waste processing facility. Only if very small amounts of copper salts are used, the waste may be flushed down the drain with lots of water.
![]()
Excess amount of thiosulfate
Dissolve some copper sulfate in one test tube and some sodium thiosulfate in another test tube. For the thiosulfate an excess amount should be used. A good guideline is to use one spatula full of copper sulfate and four of the same spatulas full of sodium thiosulfate. These two solutions are shown in the left picture. The middle picture shows the same test tubes, but now part of the contents of the right test tube is added to the left test tube. A yellow compound is formed, which remains in solution. When all thiosulfate is added to the left test tube, then the yellow color disappears and the solution becomes colorless again.



![]()
Excess amount of copper sulfate
A similar sequence of experiments is shown in the three pictures below, but now the copper sulfate solution is added slowly and carefully to the solution of the sodium thiosulfate. In the middle picture the copper sulfate was carefully added, without shaking. This shows a green liquid, floating on top of the original colorless liquid. The green color can be explained by assuming that this liquid contains the yellow complex, mixed with some excess blue aqueous copper (II) ions. The right picture shows the situation after shaking. Now a dilute solution of the yellow complex is shown. The solution of sodium thiosulfate is more dilute in this sequence of experiments (both test tubes have one spatula full of solid)



![]()
Solid copper sulfate, excess of copper sulfate
The following sequence shows addition of dissolved sodium thiosulfate to solid copper sulfate. The results again are very similar to the things shown in the two sequences above. The left picture shows the amounts of copper sulfate and sodium thiosulfate used. The middle picture shows the sodium thiosulfate, dissolved in some water. The right picture shows that half the amount of solution of sodium thiosulfate is added to the solid copper sulfate and most of the copper sulfate has dissolved already. A green mix of blue aqueous copper (II) ions and yellow copper-thiosulfate complex is shown. The liquid remains clear, even if all copper sulfate has dissolved. On adding the rest of the thiosulfate solution, the liquid in the left test tube becomes yellow again, as shown in the two sequences above.



![]()
Solid copper sulfate and solid sodium thiosulfate
Things get really interesting, when a mixture of solid copper sulfate and solid sodium thiosulfate is used and some water is added. Then a bright yellow precipitate is formed, which surprisingly does not dissolve on dilution with much more water. The top left picture shows the solid copper sulfate and sodium thiosulfate mixed. The top right picture shows the same test tube, with a small amount of distilled water added. A bright yellow, somewhat greenish, precipitate is formed. The bottom row of pictures shows the same test tube, with strong dilution of the liquid. The precipitate does not dissolve. In due time (a few hours), it slowly settles at the bottom of the test tube. The fact that the yellow compound does not dissolve probably means that this compound is not the same as the yellow complex, which is formed in solution at lower concentrations.





The yellow precipitate has some interesting properties. It dissolves in excess solution of thiosulfate, as shown by the sequence of pictures, immediately below. It can be oxidized to a dark compound, as shown further below. For the sequence below, the contents of the right test tube of the previous sequence is shaken and divided quickly over two test tubes. This is shown in the top picture. The three-picture sequence shows the addition of some sodium thiosulfate to the contents of the left test tube. The bottom picture shows both test tubes again, with the colorless liquid in the left one and the settled precipitate in the right one.




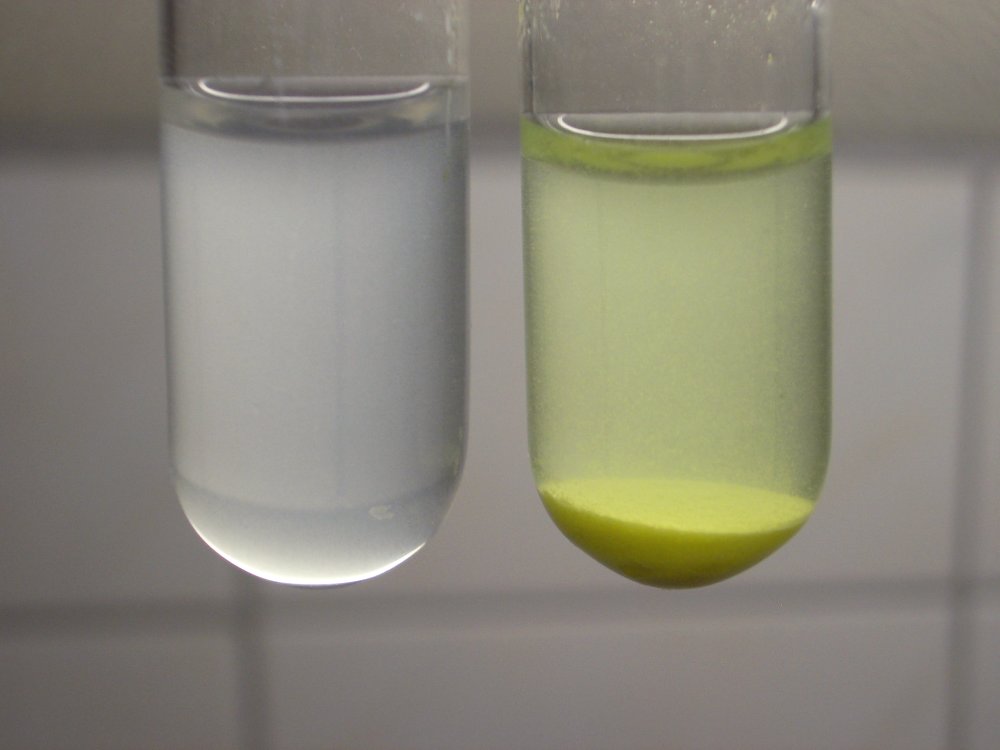
The right test tube was allowed standing for a day. In this time, more of the yellow compound was deposited on the glass in the form of a thin irregular layer. This layer extends to just below the surface of the liquid. With a little swirling, this could be easily removed from the glass. The left picture shows the contents of the test tube after a little swirling. The right picture shows the same test tube after some more swirling. The original precipitate at the bottom was not disturbed with this careful swirling around of the test tube.


On prolonged standing for several days (with open end of the test tube, but preventing dust to enter the test tube), the yellow precipitate is covered by a brown layer. The top-layer of the precipitate is converted to a dark brown compound. When the test tube is shaken now, then a dirty green/brown suspension is created, consisting of a mix of the bright yellow species and the dark brown species. Specks of the yellow species, which are sticking to the glass above the surface of the liquid, also turned brown.


The dark brown species does not dissolve, when excess sodium thiosulfate is added. A spatula full of solid sodium thiosulfate was added to the test tube, containing the mixture of the yellow and dark brown compound. The yellow compound dissolves and the dark brown compound remains visible. On standing for a longer time, the dark brown compound settles at the bottom and at the glass a somewhat milky compound is deposited. The liquid is clear, but the glass is covered by a somewhat cloudy layer. The most right picture shows this effect quite well, because of some scattered light, which makes the whole test tube look somewhat irradiant.



![]()
Discussion of the results
This sequence of experiments shows that thiosulfate can react with copper (II) in many different ways. When excess thiosulfate is used, then a colorless soluble compound is formed, independent of concentration. When more copper (II), relative to the thiosulfate, is used, then either a soluble yellow compound is formed or an insoluble yellow compound, depending on concentration. Remarkable is that the yellow insoluble compound really is insoluble and cannot be dissolved on dilution. This probably means that the two compounds are different compounds.
As shown by the last sequence of experiments, the dark brown compound, formed from the yellow compound, only is formed at the top of the yellow layer. The fact, that it is only formed at the top layer, makes air oxidation of the yellow compound a plausible explanation for the observations. It may be copper (II) oxide or copper (II) sulfide, but more investigations need to be done on this.
Remark: Further investigations (with the kind help of Frank van Parijs) have shown that the brown solid is copper sulfide, CuS. The formation of a dark thin brown layer on top of the yellow precipitate makes the explanation of air oxidation plausible, but further investigations also show, that this is not the cause of the brown precipitate. The dark brown precipitate is formed in the colorless liquid itself, very slowly. It settles at the bottom. The yellow precipitate is below the clear liquid and hence, the top part of this precipitate served as the "bottom", on which the brown precipitate settled. Careful observation shows that the undisturbed yellow precipitate also has become somewhat less bright. This can perfectly be explained, because the precipitate is immersed in the same colorless liquid as the liquid above the precipitate. Also the liquid between the bright yellow particles produced some of the brown precipitate of CuS and this makes the color of the yellow precipitate less bright.
These experiments raise the following questions:
-
What is the yellow compound in solution?
- What is the yellow precipitate?
- What is the colorless compound, when excess sodium thiosulfate is added? Is this is a copper (I) - thiosulfate compex?
- Is the dark compound indeed copper (II) oxide or copper (II) sulfide? See remarks above, it is CuS: resolved already.