


Snow in a test tube
This is a funny experiment, which is somewhat at the border of physics and chemistry. It is put in the physics section of the website, but it also could have been placed in the chemistry section.
In this experiment, a volatile solid compound, niobium pentachloride, is heated, until all of it is converted to a gas. The gas then is allowed to cool down slowly and this results in precipitation of the niobium pentachloride in the form of fine snow. The snow is formed in the gas and slowly moves downwards and settles at the glass. The snowflakes also serve as starting points, where additional niobium pentachloride sublimes from the gaseous to the solid phase. This can be regarded as formation of rime.
![]()
Required equipment:
- heat-resistant test tube
- clamp to hold the test tube
- small propane torch
- niobium pentachloride
Safety:
- The test tube is heated to well over 250 ºC. Do this experiment above a surface, which can stand the hot glass in case the test tube cracks.
- Niobium pentachloride is a very corrosive solid and reacts with water, giving off corrosive fumes of hydrogen chloride. The amount, used in this experiment, however, is small and does not impose a really high risk.
Disposal:
- Niobium only is slightly toxic for the environment. Let the test tube cool down and then add a lot of water to it and flush the waste down the drain. You probably need a brush to clean the inside of the test tube. When water is added, the insoluble Nb2O5 is formed.
![]()
Preparation of yellow gaseous NbCl5 and condensing to snow
Put a small amount (200 mg or so) of niobium pentachloride in a test tube.

Carefully heat the test tube with a propane torch. Don't use it at full blast. Slowly heat the glass and try to heat it evenly over the entire lower half part of the test tube. The test tube must be held in a clamp, don't keep it in your hand, it will become very hot, also at the higher parts, which are not heated directly.
At first, the solid melts, and an orange/red liquid is formed. This liquid already gives off yellow gaseous niobium pentachloride, but when it is heated further, you can see it boiling vigorously, and a dense deep yellow gas is produced. This is shown in the pictures below. Some of the gas may condense on the glass again as yellow/orange droplets. Also heat those again, until a yellow gas remains.


In the upper part of the test tube, a yellow/white frost will be formed. This is not a problem, just ignore that, the interesting part of the experiment is in the lower part. The frost can be removed, but that would require heating the entire test tube to over 250 ºC, which probably is not feasible, due to the clamps. When the situation is as shown below, then the experiment certainly will be successful:

Now the lower part of the test tube is completely dry and only contains the yellow gas. From this point, it just is a matter of watching the test tube, while it cools down. The effect is really neat to see. The yellow gas condenses to small solid particles, due to the rapid cooling. These particles slowly fall down. This nicely is shown in the picture below. It is amazing to see the formation of snow all over the test tube at once. Within a few seconds, there is a transition from a totally clear yellow gas, to snow, falling downwards in the test tube. The gas also becomes somewhat cloudy, just like fog and hence it is not totally clear anymore.

The left picture below shows that already quite some yellow gas has disappeared and that quite some snow has collected at the bottom of the test tube and along the left wall of the test tube. Still more snow is falling. The right picture shows the situation, almost near the end of the experiment. Only a little amount of the yellow gas remains, the air inside the test tube is almost clear again, and the snow becomes a little bit more compact.

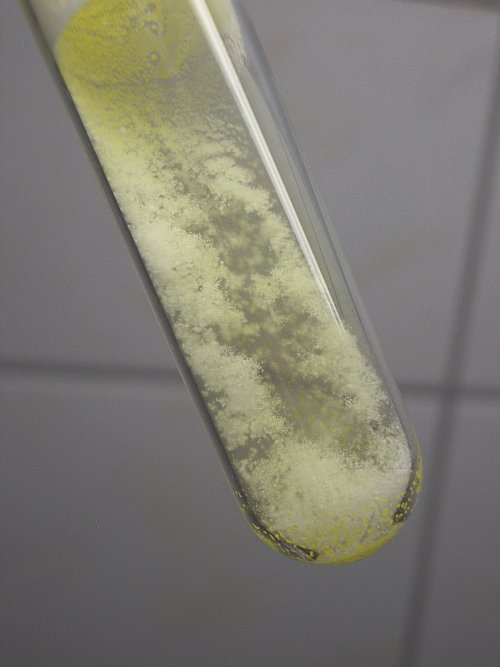
The final result after cooling down is shown below. The snow is a little sticky, the test tube can be kept somewhat upside down, as shown in the picture, without the snow moving around in the test tube. However, if the test tube is fully kept upside down, or moved quickly, then the snow does not stick to the glass anymore and then it moves around in the test tube.

Multiple videos of this experiment were made. The videos are large, but they definitely are worth the download time. The effect really is nice, especially the third video is remarkable.
- Video 1: Heating of fresh niobium chloride and allowing to cool down again
- Video 2: Reheating of the snow and rime
- Video 3: Again cooling down, now with dark background, very nice!
Download sizes are 9, 6 and 8 Mbyte for videos 1, 2 and 3.
Sometimes, the results also are somewhat different. This depends on which part of the test tube is hottest, when one stops with heating. A colder bottom parts results in a situation with no snow, as shown in this video (download size is appr. 1.7 MByte). Yet another run shows quick formation of rime, without snow falling downwards in the test tube. The formation of rime can be viewed here (download size appr. 2.4 MByte).
![]()
Discussion of results
Niobium pentachloride is a volatile compound, which melts at 205 ºC and which boils at 254 ºC. The bulk crystalline solid is yellow, the liquid is red/orange, and the gas is deep yellow. When the solid is very finely divided, then it is pale yellow or even white. A fog of very fine droplets of the liquid also is pale yellow, or even white. The precise color of the fog is hard to determine, because it can only exist in the yellow gas.
This experiment also nicely shows, that a compound, which normally can exist as liquid, also can go directly from gas to solid and vice versa. This is an effect, which we all know from winter time, when rime is present on trees, grass, etc. We also know the frost, which settles on ice-cold surfaces. A similar effect is shown in the videos, where the gas is in contact with the colder glass, on which a solid rime is formed immediately. Quick cooling down allows the gas to go to the solid state immediately. Slower cooling down first forces the gas to go to the liquid state.
The compound NbCl5 is extremely easily hydrolysed, and it might be that part of the white solid is not pure NbCl5 but NbOCl3, which is formed with humidity from the air. The compound NbOCl3 is white, and it has a melting point and boiling point similar to that of NbCl5. So, the formation of NbOCl3 might also explain why the rime and snow are white and not yellow.