


Aqueous solutions and precipitates of uranium
In aqueous solutions, uranium is said to exist in oxidation states +4, +5 and +6. Oxidation states +4 and +6 can easily be achieved, but oxidation state +5 could not be obtained, so no pictures are available of that.

The most common oxidation state in aqueous solution is +6 and in this oxidation state, uranium is neither oxidizing nor reducing. Uranium in oxidation state +4 is much less stable. It is strongly reducing and when allowed in contact with air for prolonged time in acidic solution, it slowly turns into hexavalent uranium.
![]()
Oxidation state +4
Uranium in oxidation state +4 is strongly reducing. It can be made by dissolving a uranium(VI) salt in a non-oxidizing acid like hydrochloric acid and adding zinc-powder to this. When this is done, then the liquid turns dark green and at the same time a lot of hydrogen is formed, as is shown in the opening picture of this webpage.
When all zinc has dissolved, then a green solution is obtained, which can be kept in contact with air for limited time. The green color is due to cations U4+. These ions are very prone to hydrolysis and only can exist as such in an acidic solution. In neutral solution, the liquid becomes turbid, due to formation of hydroxo-species.

After one day of standing in contact with air, the above solution had turned yellow again. The uranium(IV) was oxidized to uranium(VI) again.
When a large excess of a solution of sodium hydroxide is added, then a grey/browngreen precipitate of uranium(IV) hydroxide is formed. This precipitate also is strongly reducing and quickly turns yellow, when it is allowed to be in contact with air.


The pictures above show the precipitate of uranium(IV) hydroxide, the left picture with light falling on the precipitate and the right picture with light going somewhat through the precipitate. The precipitate is mainly grey, but under certain light conditions it has a somewhat brownish/green hue.
![]()
Oxidation state +6
This is the most common and most stable oxidation state in aqueous solution. Commercial salts of uranium always contain the metal in oxidation state +6. Uranium in oxidation state +6 forms the so-called uranyl ion, which is written as UO22+. This ion has a yellow color with a faint green tinge.

In some solids, uranyl compounds show strong fluorescence, but the aqueous solutions do not have this property. Uranyl compounds also are somewhat prone to hydrolysis, but this effect only is weak. Even in neutral solution, clear solutions of uranyl compounds can be obtained. Only when the solution is allowed to stand for prolonged time, a very thin precipitate of basic uranyl compounds is deposited.
When a solution of sodium hydroxide is added to a solution of a uranyl salt, then a yellow precipitate is formed.

This yellow precipitate can be regarded as uranyl hydroxide, UO2(OH)2. When the precipitate is shaken very violently and then allowed to settle again, then a very fine bright yellow precipitate is formed, which is hard to filter and without any visible structure.

![]()
Oxidation state +6: peroxo complex
Uranium also forms a remarkable insoluble peroxo complex, which is surprisingly stable. It can easily be prepared by adding a solution of hydrogen peroxide to a solution of a uranyl salt. It slowly is formed and simply is precipitated, forming a very fine but compact precipitate. Even from acidic solution, this complex is precipitated slowly. It does not readily dissolve in dilute mineral acids.
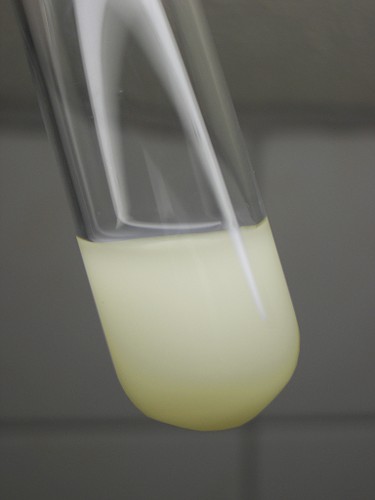
The complex has a pale yellow color (much lighter than uranyl hydroxide) and is very fine. It takes a day or so before all of the precipitate has settled at the bottom, but once it has done so it is quite compact and can easily be separated from the liquid above it. This peroxo complex has net formula UO4·2H2O. It contains one peroxo-ligand. When this precipitate is carefully heated, then it looses water and oxygen and changes to yellow UO3. The existence of this complex can be utilized to isolate uranium from other metal ions, by adding hydrogen peroxide to a somewhat acidic solution, containing uranium and other metals. The uranium precipitates, while the other metals remain in solution.
back to solutions/precipitates main page
back to miscellaneous main page