


Aqueous solutions and precipitates of gold
In aqueous solutions, gold can exist in oxidation states +1 and +3. There also is a very remarkable state of gold in oxidation state 0, which very much looks like a solution, but in reality consists of exceedingly fine particles (at nm size). These solutions have remarkable and beautiful colors and also are very strongly colored, even at ppm concentrations. Such solutions are called colloidal gold.
Gold does not appear in solution as a plain aqua-ion, it only exists in solution as a complex. With some ligands, the +1 oxidation state is very stable, with other ligand, the +3 oxidation state is stable. The +3 oxidation state of gold also easily forms an oxo-anion, the colorless ion AuO2–.
![]()
Oxidation state 0
Oxidation state 0 is the most remarkable oxidation state of gold. Normally it is in this oxidation state in the bulk state. In that oxidation state it is a nice yellow metal. In solution, however, one can make very dilute solutions, which contain clusters Aun, with n up to a few hundreds of atoms. These clusters are remarkably stable, as long as the concentration does not become too high. At too high concentrations, the clusters stick together and the gold precipitates from the solution in the form of small blue or brown particles.
The left picture below shows a solution of colloidal gold with a concentration of at most 10 μg gold per gram of solution. The middle picture shows colloidal gold with a concentration of approximately 50 μg gold per gram of solution. The right picture shows a solution with even more gold (estimated at approximately 300 μg gold per gram of solution). This solution is not stable anymore and slowly deposits a precipitate of metallic gold. Colloidal gold has a beautiful purple/blue color.



The next three pictures show the same test tubes, but now with light from behind (the light source behind the back of the camera). Here, there is more reflected light and these pictures clearly show that the appearance of colloidal gold strongly depends on how it is viewed. Light going through the liquid becomes blue, reflected light becomes red/brown. Especially the more concentrated colloidal gold solution looks quite red when viewed with light from behind.



Making such colloidal gold solutions is not hard. They can be prepared by adding a very dilute solution of a gold (III) compound (e.g. AuCl4–) to a slightly reducing solution (very dilute sodium citrate is suitable). Slowly, the colloidal gold solution is formed.
The color-properties of very finely divided gold particles are also used in photography and gold-toned images can be of striking beauty. Unfortunately, gold-toning of images is quite expensive.
Several minutes after its formation, the most concentrated colloidal gold solution started precipitating. This precipitation effect also is very nice to observe. One can clearly see the particles grow. At a certain point they become macroscopically large. The following two pictures show the most concentrated colloidal gold solution, when it starts to precipitate and then diluted 10-fold. Again with transmitted light and reflected light. The difference really is striking. It is the same liquid and the same test tube!


Finally, the following picture shows the most concentrated solution at an even later stage of precipitation. This is fascinating to watch. The particles grow and remain floating around in the liquid. Only very slowly they settle at the bottom. The particles still look blue. This means that still they are very small gold particles, clustered together in larger groups.

When a gold (III) solution is slowly reduced at much higher concentration (> 0.1% of Au by weight), then no colloidal gold is formed anymore. In that case, solid gold is formed, which either settles at the bottom or floats on the surface of the liquid (remarkably this happens easily, due to surface tension of the liquid). Below, a picture is shown of gold, formed by slow reduction of a few drops of 4% HAuCl4 with a fairly concentrated solution of KI (first a brown complex is formed, which slowly decomposes, giving the metallic gold). Formation of the little crystals of gold took several hours.

![]()
Oxidation state +1
 Oxidation
state +1 is very prone to disproportionation to the metal and the ion in the +3
oxidation state, especially in the presence of ions like chloride or bromide.
Oxidation
state +1 is very prone to disproportionation to the metal and the ion in the +3
oxidation state, especially in the presence of ions like chloride or bromide.
Some ligands, however, strongly stabilize the +1 oxidation state. These are thiosulfate and even more, cyanide. A solution of sodium cyanide or potassium cyanide is capable of dissolving metallic gold easily, provided some oxidizer is present. Oxygen from the air is perfectly suitable for this. When hydrogen peroxide is added, then it dissolves at an even higher rate. Although gold normally is very inert, the presence of cyanide ion makes it much less noble. This property of gold is exploited in extracting gold from gold ores, where the gold is mixed with a lot of rock and other debris. The gold dissolves, while the other material remains behind. The gold is recovered from such a solution by adding a strong reductor, like zinc metal.
Due to the strongly stabilizing properties of cyanide complex of gold, the following reaction is strongly favored:
4Au + 2H2O + O2 + 8CN– → 4Au(CN)2– + 4OH–
A solution of such a cyano-gold complex is colorless. The picture shows a solution of gold in a solution of NaCN, to which a few drops of dilute H2O2 are added.
![]()
Oxidation state +3
This is the most common oxidation state for gold in its compounds. The most common commercial compound of gold is the so-called "gold chloride", but in reality this compound is hydrated tetrachloroauric acid, HAuCl4·xH2O.
The solid is orange/yellow and its solutions are deep yellow. Below, a picture is shown of a 4% solution of this compound in water. The yellow color is caused by the AuCl4– ion.

When such a solution is made alkaline, then no precipitate is formed, but the solution becomes lighter and remains clear. The left picture shows half the amount of the 4% solution, with some fairly concentrated solution of NaOH added. The liquid becomes light yellow, not entirely colorless. This lightening effect is not immediate. That is shown by the right picture. The remaining part of the 4% solution was divided over two test tubes and approximately equal amounts of solution of NaOH were added. The left test tube in the right picture had NaOH added just before making the picture, the right test tube in the right picture had NaOH added several minutes earlier. Finally, both test tubes contained a very pale yellow liquid.

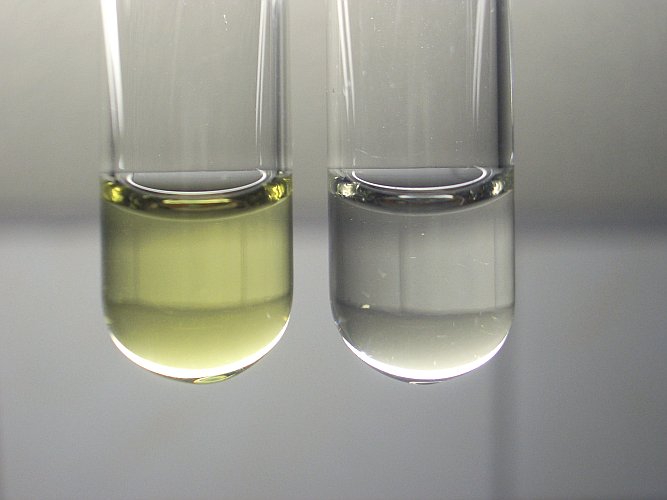
In the strongly alkaline solution, the AuCl4– ion slowly looses its chloride ligands and they are replaced by hydroxide-ligands, followed by subsequent expelling of water molecules:
AuCl4– + 4OH– ↔ Au(OH)4– + 4Cl–
Au(OH)4– ↔ AuO2– + 2H2O
Finally, the solution contains colorless aurate ions, AuO2–, with a very low concentration remaining of chloro-ligand ions (these are responsible for the remaining very weak yellow color). The higher the pH, the more strongly the equilibrium moves to the right side of the equations.
back to solutions/precipitates main page
back to miscellaneous main page