


Riddle: Titanium and fluoride
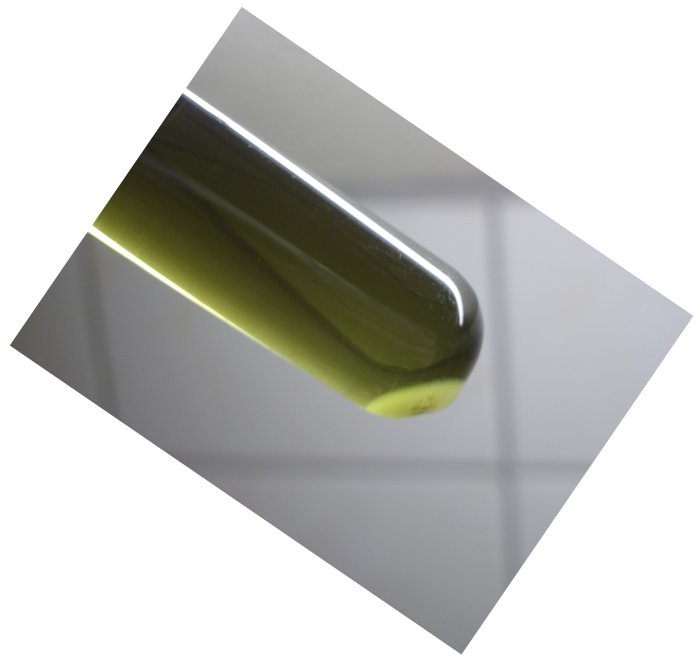
It is known that titanium in its +3 oxidation state forms
purple/blue cations, which can best be described as Ti(H2O)63+.
A picture of the deep
 blue/purple
compound is shown here. It was prepared by dissolving some titanium
metal in concentrated hydrochloric acid in a loosely stoppered test tube. It took a few
days, before such a nice deep purple/blue liquid was formed. When the purple
solution is partially oxidized, then a lighter purple solution is created, until
finally the solution becomes colorless on complete oxidation of the purple
solution. This happens on addition of an oxidizer, such as ammonium persulfate,
but also on standing in contact with air for several days.
blue/purple
compound is shown here. It was prepared by dissolving some titanium
metal in concentrated hydrochloric acid in a loosely stoppered test tube. It took a few
days, before such a nice deep purple/blue liquid was formed. When the purple
solution is partially oxidized, then a lighter purple solution is created, until
finally the solution becomes colorless on complete oxidation of the purple
solution. This happens on addition of an oxidizer, such as ammonium persulfate,
but also on standing in contact with air for several days.
With fluoride, however, titanium apparently forms a green complex, which has the remarkable property to form a deep brown complex on partial oxidation and which is converted to a colorless compound on complete oxidation. The colorless compound is the fairly well-known hexafluorotitanate (IV), which can be written as TiF62–. Salts of this anion can be purchased commercially and an aqueous solution of the acid H2TiF6 is also available commercially.
Textbooks do not mention the existence of a green complex with fluoride nor the formation of a dark brown compound with the partially oxidized green compound. Probably, the green compound is a titanium (III) coordination complex with fluoride and the dark brown compound may be a mixed valence titanium complex, with titanium in its +3 and its +4 oxidation state. This behavior resembles the behavior of copper solutions, which contain both copper (I) and copper (II) at the same time, in the presence of chloride. See riddle on copper (I) and copper (II).
![]()
![]() Required
chemicals:
Required
chemicals:
-
titanium metal, 99.9%. The metal used in this experiment is quite pure. An alloy, containing other metals besides titanium should not be used, because of interference and possible formation of colored compounds, which may affect the observed outcome of the experiment.
-
dilute hydrochloric acid, approximately 10% by weight
-
sodium fluoride
-
ammonium persulfate
![]() Required
equipment:
Required
equipment:
-
test tubes
-
stopper
- bunsen burner or equivalent, for heating the test tubes
![]() Safety:
Safety:
-
In this experiment a small amount of hydrofluoric acid is produced. Probably, this acid does not enter the air at any appreciable amount, but it is best to perform the experiment in a well-ventilated room.
-
In this experiment some hydrofluoric acid is formed in a dilute solution of hydrochloric acid. Hydrofluoric acid is insidiously toxic and very painful on the skin. I'm not sure, whether the low-concentration solutions, produced here are that dangerous, but just to be sure: be careful not to get any liquid with HF in it on the skin!
-
Hydrochloric acid (10%) is corrosive.
- Sodium fluoride is toxic and may irritate the skin.
![]() Disposal:
Disposal:
-
The chemicals, used in this experiment, can be flushed down the drain with a large amount of water. NaF and HF are toxic, but when diluted sufficiently strongly, then the small amounts, used in this experiment are not of real concern. Most tooth-pastes also contain 0.1% NaF and these also are flushed down the drain. Titanium metal and its compounds are not particularly toxic for the environment.
![]()
Dissolving titanium metal in dilute HCl and NaF
![]() Add a very small amount of titanium metal to a few ml of dilute hydrochloric
acid with approximately 10% HCl by weight. When this is done, then one can
observe that the metal does not dissolve. When the liquid is heated, then still
the metal hardly dissolves.
Add a very small amount of titanium metal to a few ml of dilute hydrochloric
acid with approximately 10% HCl by weight. When this is done, then one can
observe that the metal does not dissolve. When the liquid is heated, then still
the metal hardly dissolves.
![]() Add a spatula full of sodium fluoride to the still hot
liquid. This solid fairly easily dissolves in the dilute hydrochloric acid. As
soon as the sodium fluoride is added, the metallic titanium is dissolved quickly
and a lot of gas is produced. This gas is hydrogen gas. This can be tested
easily, by
loosely stoppering the test tube for a while and then keeping a flame in front
of the test tube. A 'whoop' sound can be heard from the burning hydrogen gas.
While the titanium metal dissolves, a green compound is formed. The following
pictures show the test tube, a short time after the sodium fluoride is added,
after a second time of heating and shaking, and finally, when almost all titanium
metal is dissolved.
Add a spatula full of sodium fluoride to the still hot
liquid. This solid fairly easily dissolves in the dilute hydrochloric acid. As
soon as the sodium fluoride is added, the metallic titanium is dissolved quickly
and a lot of gas is produced. This gas is hydrogen gas. This can be tested
easily, by
loosely stoppering the test tube for a while and then keeping a flame in front
of the test tube. A 'whoop' sound can be heard from the burning hydrogen gas.
While the titanium metal dissolves, a green compound is formed. The following
pictures show the test tube, a short time after the sodium fluoride is added,
after a second time of heating and shaking, and finally, when almost all titanium
metal is dissolved.



The three pictures show that the green compound is nice bright green. After shaking the test tube in contact with air, the color has darkened somewhat and has shifted towards brown. This is due to oxidation of the green compound by oxygen from the air. Below, more evidence for this hypothesis is shown.
![]()
Oxidation of green compound by oxygen from air
![]() Dilute the liquid with water approximately 2.5 times and let stand for a few
hours with the test tube stoppered. After these hours, the liquid is clear, but
near the surface, the liquid is brown.
Dilute the liquid with water approximately 2.5 times and let stand for a few
hours with the test tube stoppered. After these hours, the liquid is clear, but
near the surface, the liquid is brown.
The following two pictures show the diluted liquid, after a few hours of standing and after some careful swirling of the test tube:


The left picture clearly shows the brown layer near the surface. The second picture shows that the entire liquid has become somewhat more brown after swirling of the test tube. The only source of the brown compound can be oxidation of the green compound by oxygen from the air. This can be safely assumed, because of the fact that only near the surface, such a brown color can be observed.
![]() Another phenomenon, which can be observed in the pictures,
is that there is a thin layer of a gelatinous white precipitate at the bottom of
the test tube. This probably is due to reaction of hydrofluoric acid with the
glass of the test tube, resulting in formation of flocculent hydrous SiO2
on dilution of the liquid. The picture below shows the precipitate more clearly,
with the test tube kept at an angle of approximately 40 degrees:
Another phenomenon, which can be observed in the pictures,
is that there is a thin layer of a gelatinous white precipitate at the bottom of
the test tube. This probably is due to reaction of hydrofluoric acid with the
glass of the test tube, resulting in formation of flocculent hydrous SiO2
on dilution of the liquid. The picture below shows the precipitate more clearly,
with the test tube kept at an angle of approximately 40 degrees:
![]()
Partial and complete oxidation of green compound
![]() A small part of the green/brown solution is
transferred to a separate test tube, in which first some solid ammonium
persulfate was put. This results in partial oxidation of the green compound at
the upper part of the liquid and complete oxidation at the lower part. When the
liquid is allowed to stand for a while, then the persulfate-solution diffuses
upwards and the layer of completely oxidized titanium-compound becomes thicker.
The partially oxidized compound is dark brown, while the completely oxidized
compound is colorless. The partially oxidized compound probably is a highly
colored titanium (III) / titanium (IV) mixed oxidation state complex.
A small part of the green/brown solution is
transferred to a separate test tube, in which first some solid ammonium
persulfate was put. This results in partial oxidation of the green compound at
the upper part of the liquid and complete oxidation at the lower part. When the
liquid is allowed to stand for a while, then the persulfate-solution diffuses
upwards and the layer of completely oxidized titanium-compound becomes thicker.
The partially oxidized compound is dark brown, while the completely oxidized
compound is colorless. The partially oxidized compound probably is a highly
colored titanium (III) / titanium (IV) mixed oxidation state complex.


The two pictures above show the dark brown complex and the colorless liquid at the bottom, with some solid ammonium persulfate at the bottom. The right picture shows the same test tube somewhat later. The colorless layer of completely oxidized titanium-compound has become thicker.
![]() When the test tube is swirled carefully and the
persulfate is mixed throughout the complete liquid, then the brown compound
quickly - but not instantaneously - disappears. This is shown by the two pictures below.
When the test tube is swirled carefully and the
persulfate is mixed throughout the complete liquid, then the brown compound
quickly - but not instantaneously - disappears. This is shown by the two pictures below.


![]()
Conversion of purple titanium (III) to green fluoro complex
The green fluoro complex also can be made by adding fluoride to the purple solution, obtained from dissolving titanium metal in concentrated hydrochloric acid. In this part of the experiment the following is done:
![]() Dissolve some titanium metal in concentrated
hydrochloric acid in a loosely stoppered test tube, such that not much air can
enter the test tube. The metal is left in the acid for several days, such that a
deep purple/blue solution is obtained, as shown at the top of this page.
Dissolve some titanium metal in concentrated
hydrochloric acid in a loosely stoppered test tube, such that not much air can
enter the test tube. The metal is left in the acid for several days, such that a
deep purple/blue solution is obtained, as shown at the top of this page.
![]() Dilute the liquid with a lot of water. This results in
a light purple solution. Note the color shift. On dilution, the bluish color
disappears and the liquid becomes more purple.
Dilute the liquid with a lot of water. This results in
a light purple solution. Note the color shift. On dilution, the bluish color
disappears and the liquid becomes more purple.
![]() Add a spatula of solid sodium fluoride. The liquid
becomes light green at once and remains clear.
Add a spatula of solid sodium fluoride. The liquid
becomes light green at once and remains clear.
![]() Add a small amount of ammonium persulfate. This
results in formation of a brown compound. The change of color is not immediate,
as was the case for the transition from purple to green. It takes a few minutes
before the liquid obtains its final brown color.
Add a small amount of ammonium persulfate. This
results in formation of a brown compound. The change of color is not immediate,
as was the case for the transition from purple to green. It takes a few minutes
before the liquid obtains its final brown color.
![]() Add more ammonium persulfate. The liquid in the test
tube slowly becomes colorless. All titanium is oxidized to its +4 oxidation
state and hence, the liquid becomes colorless.
Add more ammonium persulfate. The liquid in the test
tube slowly becomes colorless. All titanium is oxidized to its +4 oxidation
state and hence, the liquid becomes colorless.
Below, three pictures of the reactions are shown. At the left the diluted solution of titanium (III) chloride in very dilute hydrochloric acid is shown. In the middle, the same liquid is shown, immediately after adding the sodium fluoride with some shaking. At the right, the same test tube is shown, a few minutes after the ammonium persulfate is added. The fourth step with the colorless liquid is not shown here.



This experiment shows that the purple titanium(III)-aqua complex is labile. The aqua ligands apparently can be replaced easily by fluoro ligands. This experiment gives strong evidence that the green compound indeed is a fluoro-titanium (III) complex, but the exact nature still is not clear.
The last test tube shows the mixed valence titanium (III/IV) compound. The composition of this is a total mystery (at least for me).
![]()
Discussion of the results
Literature mentions the existence of a very stable colorless complex TiF62–. This experiment, however, shows formation of a completely different compound. It is assumed that the green compound, formed when titanium metal dissolves in HCl/NaF solution is a titanium (III) complex of fluoride and possibly chloride. It is not a complex of chloride only, because of the fact that titanium gives a blue/purple compound with chloride only. What is the composition of this green compound?
When the green compound is oxidized partly, then a dark brown compound is formed. This is shown already in the first part of the experiment, in which the titanium metal is dissolved. When the test tube with the green liquid is heated and shaken in a test tube, filled with air, then a brown compound is formed. The color shifts from green to brown/green.
The oxidation to a brown compound is even more pronounced when the diluted green liquid is allowed to stand for a few hours. At the surface, the liquid becomes brown. The only explanation, which can be given here is that oxygen from the air is absorbed by the liquid and that this results in formation of a brown compound.
The most striking result is given in the last two parts of the experiment. When the green liquid is exposed to a small amount of ammonium persulfate, then it becomes brown. When more ammonium persulfate is allowed to mix into the liquid, then it becomes colorless. The colorless liquid most likely will contain the stable TiF62– complex. The formation of the intermediate dark brown compound is quite surprising, but because of the fact that this is intermediate between the green liquid and the colorless liquid, the best explanation is that this is a mixed oxidation state complex of titanium. This complex apparently does not depend on the oxidizer, oxygen from the air and persulfate yield the same result.
Remarks:
- Sodium persulfate or potassium persulfate instead of ammonium persulfate yield the same result.
- Letting the green liquid stand in contact with air for a longer time results in total brown coloration of the liquid.
- When hydrochloric acid is replaced by 30% nitric acid with the amount of NaF unchanged, then the titanium metal still dissolves, but then no green liquid is formed. The liquid then becomes colorless and somewhat white/cloudy and the metal dissolves more slowly. Apparently, the oxidizing properties of 30% nitric acid prevent formation of a titanium (III) compound and hence, no colored complex can be observed. In 30% nitric acid with NaF, only colorless TiF62- complex is formed.