


Riddle: Oxidation of thiocyanate by chlorine
This is an experiment, in which thiocyanate is oxidized by chlorine. The thiocyanate is added to a liquid, with dissolved chlorine in it and with free chlorine above it. Another set of experiments is performed with solid sodium thiocyanate in humid chlorine gas.
Questions, raised during these experiments, are (1) what is the brown/red compound, which is formed in solution and (2) what is the yellow or orange solid, which is formed on reaction of solid thiocyanate with chlorine?
![]()
![]() Required
chemicals:
Required
chemicals:
- sodium thiocyanate
- dilute hydrochloric acid, approximately 10% HCl by weight
- calcium hypochlorite, or bleach without detergent and perfume additives with 10% active chlorine
![]() Required
equipment:
Required
equipment:
- test tube
- 100 ml erlenmeyer or any other glass flask or bottle
- stopper
- a piece of glass or something else, needed for loosely capping the erlenmeyer or
![]() Safety:
Safety:
- In this experiment a few tens of ml of chlorine gas is produced. This is sufficient for getting a really tough cough. If no proper ventilation is available (standard kitchen exhaust is not sufficient), then the experiment MUST be done outside.
- Hydrochloric acid (10%) is corrosive.
- Calcium hypochlorite is a strong oxidizer and is corrosive. The reaction between calcium hypochlorite and hydrochloric acid is vigorous, so do not scale up the experiment with larger amounts.
- Bleach is corrosive.
![]() Disposal:
Disposal:
-
The chemicals, used in this experiment, can be flushed down the drain with a large amount of water.
![]()
A brown/red reaction product, dissolved in water
Prepare a small amount of chlorine gas, by adding
a spatula full of calcium hypochlorite to approximately 10 ml of dilute
hydrochloric acid. Do this in a
 small
bottle or erlenmeyer, which must be covered or loosely capped. After this step,
a small amount of chlorine gas is prepared, mixed with some air, as shown in the
picture. The acidic liquid contains dissolved chlorine. Instead of calcium
hypochlorite, one to two ml of bleach may be used.
small
bottle or erlenmeyer, which must be covered or loosely capped. After this step,
a small amount of chlorine gas is prepared, mixed with some air, as shown in the
picture. The acidic liquid contains dissolved chlorine. Instead of calcium
hypochlorite, one to two ml of bleach may be used.
Next, add a spatula full of solid sodium thiocyanate to the gas and liquid. Try to avoid that some solid is sticking to the glass. Another option is to take a spatula full of sodium thiocyanate and dissolve this in 1 ml of water and add this solution to the liquid. After adding the sodium thiocyanate, the erlenmeyer or flask must be quickly covered again, otherwise too much of the chlorine is lost. Swirl the flask, such that the thiocyanate is mixed well through the liquid. This results in quick absorption of the chlorine gas into the liquid. The liquid becomes light brown/red. This brown/red compound is one of the reaction products of thiocyanate with chlorine. Besides this, a faint white fume is produced. The two pictures below show the light brown/red compound and the faint white fume.


Surprising result of this experiment is the formation of the brown/red compound. What is this compound? In literature, this compound is not mentioned anywhere. Oxidation products of SCN–, mentioned in literature are (SCN)2, CN– and SO42-. These, however, all are colorless. As shown by a control-experiment with bromine-water and thiocyanate, a colorless oxidation product indeed is formed, when thiocyanate is oxidized by bromine, but with chlorine a colored oxidation product is formed.
![]()
A solid insoluble reaction product
Thiocyanate can also be oxidized by chlorine to a solid, insoluble reaction product, with a color, ranging from bright yellow to bright orange/brown. This solid insoluble reaction product is quite stable and can be kept for weeks without visible changes. The procedure is as follows:
Prepare some chlorine gas in a test tube, by
adding a small spatula full of calcium hypochlorite or 1 ml of bleach to a few
ml of dilute hydrochloric acid. This step of the experiment must absolutely be
done in a good fume

 hood,
or it must be done outside. A fairly large amount of chlorine gas will escape
into the air. Inside the test tube fairly pure chlorine gas is prepared. This is
shown in the left picture. The test tube must be loosely stoppered with a rubber
stopper as long as bubbles of chlorine gas are produced. If bubbling stops, then
the rubber stopper can be pressed firmly into the test tube. Shake a few times
to clean the glass of the test tube, in order to remove small pieces of calcium
hypochlorite or droplets of bleach. Add a small spatula full of sodium
thiocyanate to the chlorine gas, but now add this to the top of the test tube,
such that all solid sticks to the glass. Immediately after adding the solid, the
test tube must be stoppered tightly again. The solid quickly reacts with the
chlorine. It becomes warm and changes color. It first turns bright yellow. When
kept in contact for a longer time, it becomes orange/brown.
hood,
or it must be done outside. A fairly large amount of chlorine gas will escape
into the air. Inside the test tube fairly pure chlorine gas is prepared. This is
shown in the left picture. The test tube must be loosely stoppered with a rubber
stopper as long as bubbles of chlorine gas are produced. If bubbling stops, then
the rubber stopper can be pressed firmly into the test tube. Shake a few times
to clean the glass of the test tube, in order to remove small pieces of calcium
hypochlorite or droplets of bleach. Add a small spatula full of sodium
thiocyanate to the chlorine gas, but now add this to the top of the test tube,
such that all solid sticks to the glass. Immediately after adding the solid, the
test tube must be stoppered tightly again. The solid quickly reacts with the
chlorine. It becomes warm and changes color. It first turns bright yellow. When
kept in contact for a longer time, it becomes orange/brown.
When the test tube is shaken, then the solid is rinsed from the glass and is dispersed throughout the liquid. It does not dissolve. Below, two different samples are shown, one with the orange/brown solid, and one with a yellow sample. The yellow sample had aged for about a week, without noticeable change.

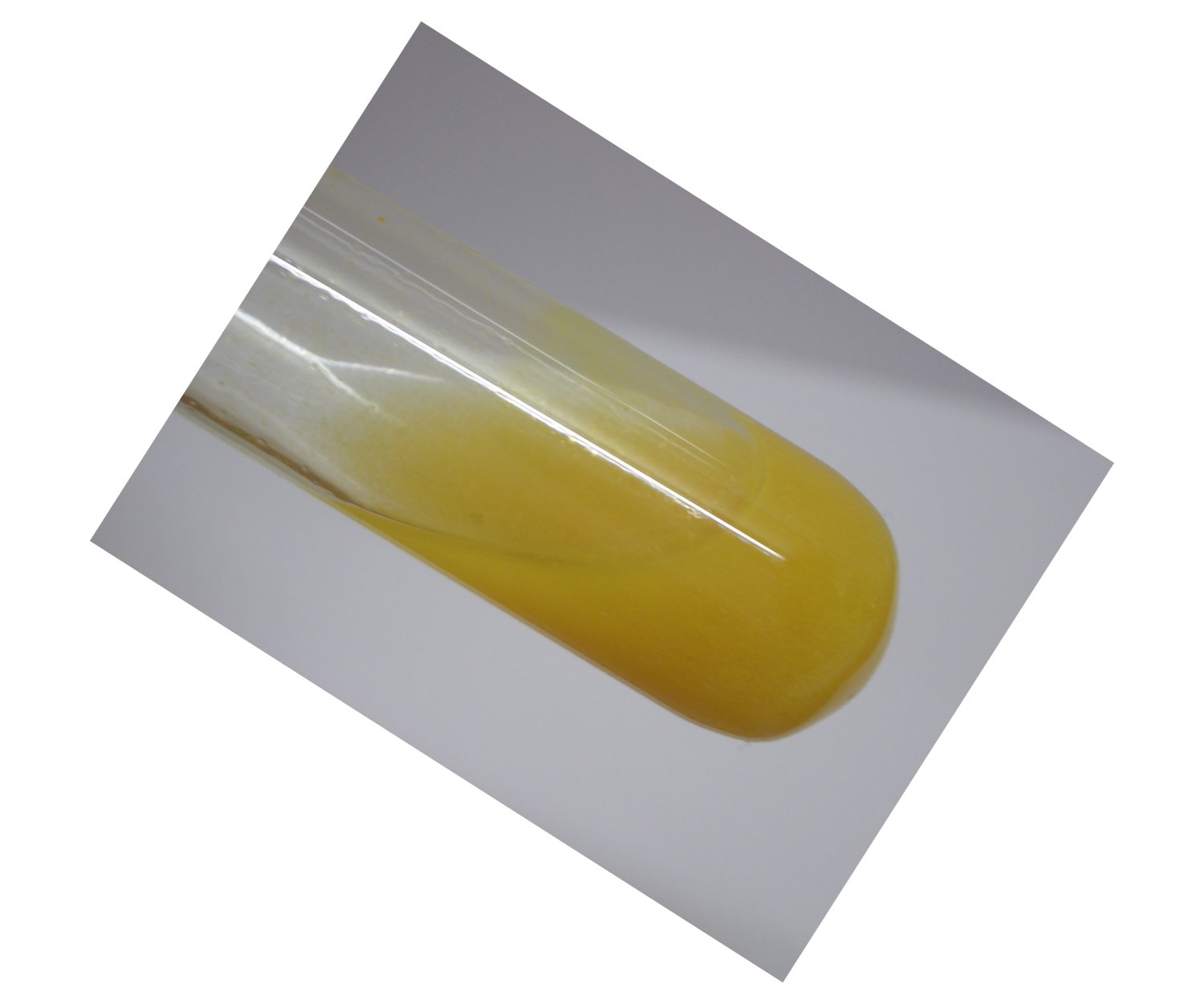
Here again, the reaction product is quite remarkable. It is no sulphur. Sulphur has a much lighter color, especially when it is precipitated from an aqueous solution (e.g. compare with sodium thiosulfate, acidified with some acid). The solid is stable towards acid. The liquid in which the solid is dispersed is quite acidic, because it is derived from dilute hydrochloric acid. When a lot of water is added to the liquid with the yellow or brown/orange solid, then the solid still does not dissolve, nor does it change noticeably.
![]()
Discussion of the results
Remarkable about this experiment is that the experimental conditions greatly affect the final reaction products. When solid thiocyanate is added to the liquid, then the reddish compound in solution is formed, when solid thiocyanate is not added to the liquid, but allowed to react as solid with gaseous chlorine, then an insoluble orange or yellow compound is formed. The reason for the difference may be the acidity in the liquid or that with thiocyanate in the solid phase the reaction with chlorine proceeds further than with thiocyanate in the dissolved phase.
Remark: This experiment can also be performed with ammonium thiocyanate or potassium thiocyanate. The results are similar.