


Strong contrasts -- green gas and red smoke
A remarkable compound (or mix of compounds) is prepared in this experiment. At high dilution, the vapor of this compound has a distinct green color, which shifts towards yellow on stronger heating. When the compound is poured at in cold air, then it immediately turns into a red/orange smoke.

When it condenses on a glass wall, then it forms a dark brown solid or a red/brown liquid. The precise nature of this compound is not entirely clear to me.
![]() Beware, vanadium-containing vapors are released in the air and avoid breathing
any of the vapors or smoke.
Beware, vanadium-containing vapors are released in the air and avoid breathing
any of the vapors or smoke.
![]()
![]() Required
chemicals:
Required
chemicals:
-
vanadium pentoxide
-
phosphorus pentachloride
![]() Required
equipment:
Required
equipment:
-
heat resistant test tube
- bunsen burner or blow torch
![]() Safety:
Safety:
- Vanadium pentoxide is toxic and usually appears as a dry free-flowing powder. Avoid inhalation of dust.
- Phosphorus pentachloride is very corrosive and gives nasty fumes of HCl in contact with air. Handle this product with care in a well-ventilated area, or in a fume hood.
![]() Disposal:
Disposal:
- Due to the vanadium content, do not flush the waste down the drain. Bring the liquid remains of this experiment to a proper waste processing facility.
![]()
Procedure for performing the experiment
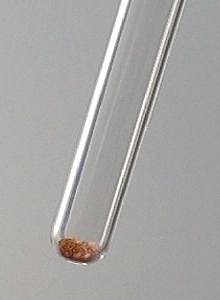
![]() Put a spatula full of phosphorus pentachloride in a perfectly
dry test tube and add a small amount of vanadium pentoxide.
Put a spatula full of phosphorus pentachloride in a perfectly
dry test tube and add a small amount of vanadium pentoxide.
![]() Swirl the test tube, such that both powders are well mixed.
The resulting mix is orange/brown and it seems to darken very slowly.
Swirl the test tube, such that both powders are well mixed.
The resulting mix is orange/brown and it seems to darken very slowly.
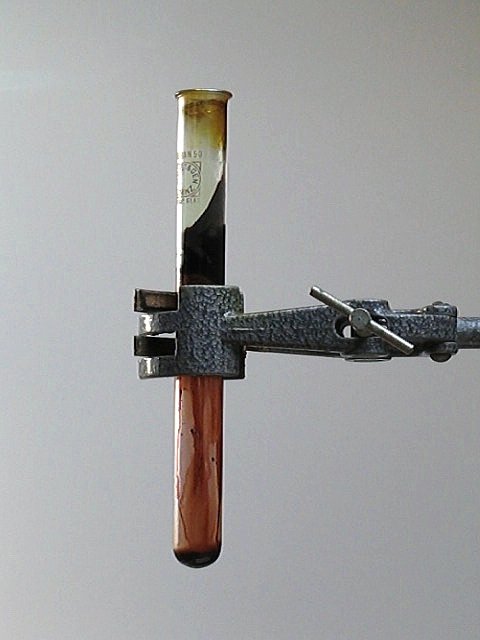
![]() Carefully heat the test tube with a bunsen flame, or with a propane torch. This
results in formation of a green vapor, which condenses to a dark brown/red solid
or liquid, which settles on the cooler parts of the glass wall.
Carefully heat the test tube with a bunsen flame, or with a propane torch. This
results in formation of a green vapor, which condenses to a dark brown/red solid
or liquid, which settles on the cooler parts of the glass wall.

On strong heating, a beautiful green/yellow gas is formed, which has a fairly intense color. The following picture shows this gas:

A video is available in which the formation of the green gas is shown, and it also shows the formation of the red/brown smoke, which settles on the glass a red liquid or a very dark brown solid: formation of green vapor (download size just over 7 MByte).
![]() After
the heating and cooling down again, the upper part of the test tube is covered
with a dark brown solid. When this is gently heated again, then a pale green
vapor is formed (which is more green and less yellow than the gas shown above),
which condenses to a dark red liquid in the lower part of the test tube.
After
the heating and cooling down again, the upper part of the test tube is covered
with a dark brown solid. When this is gently heated again, then a pale green
vapor is formed (which is more green and less yellow than the gas shown above),
which condenses to a dark red liquid in the lower part of the test tube.
When the heating becomes somewhat stronger, then the color of the vapor shifts towards yellow again and the color becomes more intense. All of this is shown in a second video, which has a download size of almost 8 MByte.
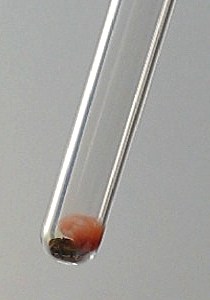
A more detailed picture of the red liquid, sticking to the glass and some of the collected liquid at the bottom is shown below. The picture also shows that the test tube is filled with a red smoke. This is not a red gas/vapor, but it really is a red smoke.

![]() When the red liquid is heated again strongly, then again the
green gas is formed. On cooling down, the vapor becomes more and more pale and
the color shifts more towards pure green as shown below. Still, the test tube is
very hot in the bottom part and certainly should not be touched.
When the red liquid is heated again strongly, then again the
green gas is formed. On cooling down, the vapor becomes more and more pale and
the color shifts more towards pure green as shown below. Still, the test tube is
very hot in the bottom part and certainly should not be touched.

![]() A beautiful effect is obtained when the green vapor is poured
into the air. It condenses to a red/orange smoke as demonstrated by a
third video
(download size almost 3 MByte). The picture at the top of this website shows
some of the orange smoke.
A beautiful effect is obtained when the green vapor is poured
into the air. It condenses to a red/orange smoke as demonstrated by a
third video
(download size almost 3 MByte). The picture at the top of this website shows
some of the orange smoke.
![]() Finally, when the still fairly hot test tube is kept under a
running tap, then quickly a red smoke is formed in the test tube. When some
water is added to the red smoke, then a yellow/green liquid is obtained.
Finally, when the still fairly hot test tube is kept under a
running tap, then quickly a red smoke is formed in the test tube. When some
water is added to the red smoke, then a yellow/green liquid is obtained.

When the test tube is shaken, then all smoke quickly is absorbed by the water and all dark solid material, sticking to the glass, also quickly is absorbed and a turbid yellow/green liquid remains.

The liquid has a clear green hue and this can only be due to the presence of some blue vanadyl ion (which contains vanadium in the +4 oxidation state). The mix of yellow/brown vanadium pentoxide and some blue vanadyl gives the yellow/green color. The turbidity is due to the low solubility of the vanadium pentoxide.
![]()
Discussion of results
What happens in this experiment cannot easily be explained from basic knowledge of certain compounds. Literature describes the following compounds of vanadium:
- VOCl3: Pale yellow liquid, yellow-green vapor
- VCl4: Red/brown liquid
- VOCl2: Bright green crystalline volatile solid
The observations in this experiment do not really match any of these compounds. The dark brown solid, which settles at the glass does not match the descriptions. The red liquid, formed in the second step of the experiment could be VCl4, but this does not match with its behavior on addition of water. VCl4 gives bright blue solutions, when water is added, and such behavior could not be reproduced.
Most likely, initially the following reaction occurs:
V2O5 + 3PCl5 → 2VOCl3 + 3POCl3
From this reaction, other compounds are formed. If only VOCl3 and POCl3 were formed, then the liquid would become pale yellow and no dark brown solid and/or liquid would be formed. The dark brown solid also is a mystery compound. It cannot be VCl4, because that has a melting point of –28 °C. Could this be a mixed phosphorus/vanadium compound?
The green vapor/gas could be VOCl3, but this is not really sure. Literature indeed mentions a green/yellow color for the vapor of VOCl3, but nothing is said about the intensity of the color and the effect of shifting towards yellow, when the concentration of the gas becomes higher and/or when the temperature becomes higher.
The red/orange smoke almost certainly is V2O5 or some hydrated form of this. It is well-known that highly condensed species of general formula (V2O5∙xH2O)n can have deep red colors.
This V2O5 is formed by hydrolysis in (humid) air:
2VOCl3 + (3+x)H2O → V2O5∙xH2O + 6HCl