


Tin chloride and iodide
Tin in the +2 oxidation state forms a colored precipitate with iodide. The color of the precipitate depends on the conditions of the experiment. The precipitate is tin (II) iodide, but the cause of the different colors is not clear. It has to do with the concentration of the reactants.
This is quite a remarkable experiment, because of the bright colors involved and the unfamiliar nature of the compound formed.
![]()
![]() Required
chemicals:
Required
chemicals:
-
tin (II) chloride
-
potassium iodide
-
dilute hydrochloric acid, approximately 10% by weight
![]() Required
equipment:
Required
equipment:
-
clean glass plate, preferably a watch glass.
![]() Safety:
Safety:
- dilute hydrochloric acid is corrosive
![]() Disposal:
Disposal:
- The amounts, used in this experiment are very small (just a few drops) and the compounds used are not particularly toxic. The waste can be flushed down the drain with a lot of water.
![]()
Procedure for performing the experiment
![]() Take a
small pinch of solid potassium iodide and a small pinch of solid tin (II)
chloride. Put these solids a few cm apart. Put a few drops of water on each of
the chemicals and carefully wet both of them. Assure that the liquids do not yet
come in contact. The result looks like this:
Take a
small pinch of solid potassium iodide and a small pinch of solid tin (II)
chloride. Put these solids a few cm apart. Put a few drops of water on each of
the chemicals and carefully wet both of them. Assure that the liquids do not yet
come in contact. The result looks like this:

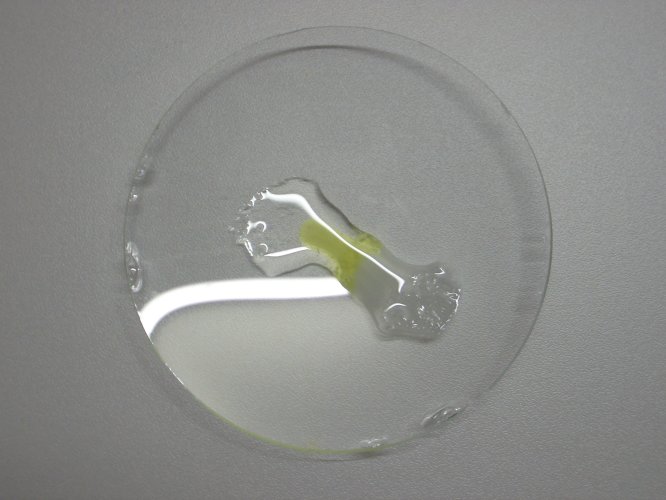
The clear drop is a highly concentrated solution of potassium iodide and the somewhat turbid solution is a concentrated solution of tin (II) chloride, somewhat hydrolysed, with some solid tin (II) chloride left.
![]() Let
liquids slowly flow towards each other and allow contact between the liquids. If
a flat piece of glass is used, then the liquids can be brought into contact with
a plastic spatula. When this is done, then at first a yellow precipitate is
formed, but slowly an orange crystalline solid is formed, at the places of
highest concentration of tin (II) chloride:
Let
liquids slowly flow towards each other and allow contact between the liquids. If
a flat piece of glass is used, then the liquids can be brought into contact with
a plastic spatula. When this is done, then at first a yellow precipitate is
formed, but slowly an orange crystalline solid is formed, at the places of
highest concentration of tin (II) chloride:
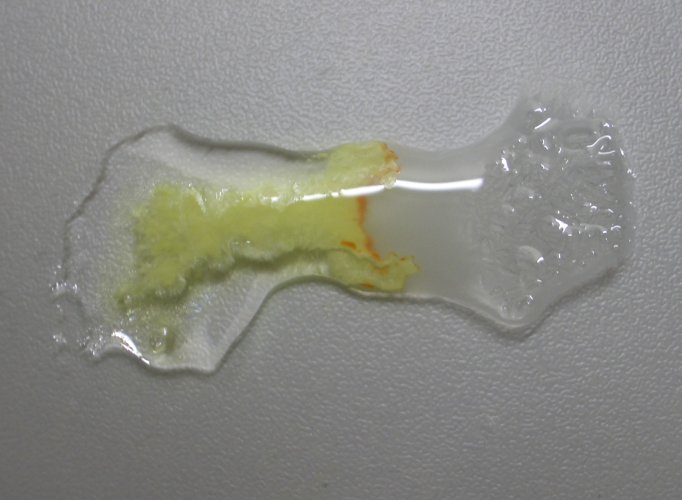
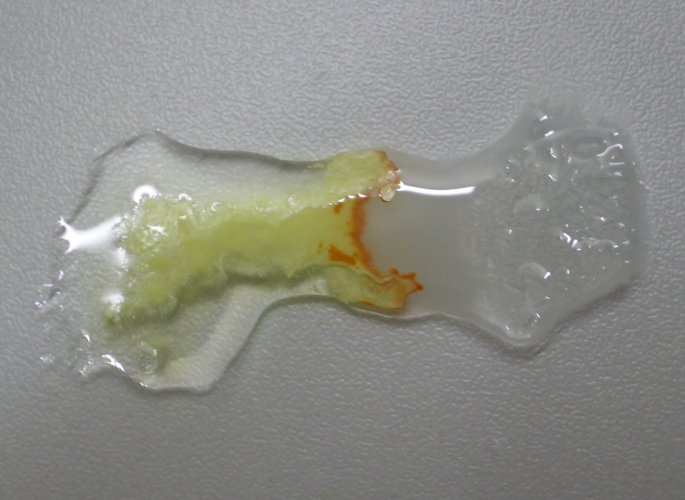
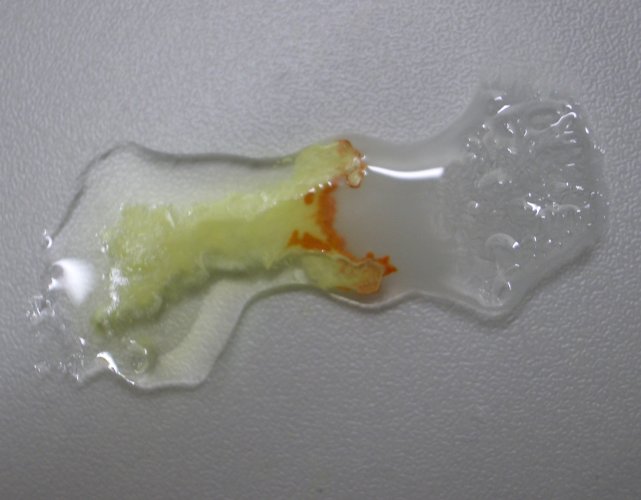
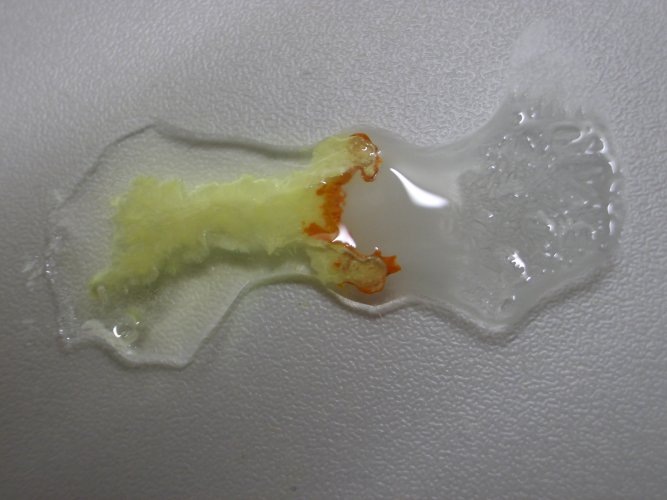
![]() Allow
the liquids to stand for a few minutes. The presence of the orange compound
becomes pronounced more and more:
Allow
the liquids to stand for a few minutes. The presence of the orange compound
becomes pronounced more and more:
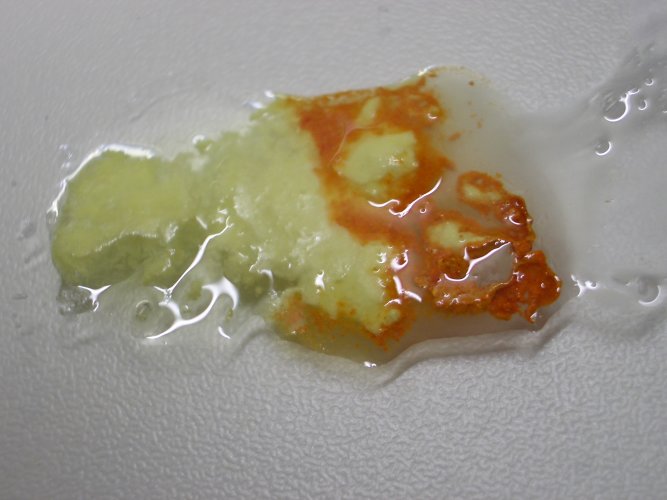
![]() Add a small amount of dilute hydrochloric acid to the
mixture. The mixture slowly dissolves. When a spatula is used to mix up things a
little, then within a minute or two, the complete mixture is dissolved and the
liquid becomes colorless and a little turbid (due to hydrolysis of tin (II)
ions):
Add a small amount of dilute hydrochloric acid to the
mixture. The mixture slowly dissolves. When a spatula is used to mix up things a
little, then within a minute or two, the complete mixture is dissolved and the
liquid becomes colorless and a little turbid (due to hydrolysis of tin (II)
ions):



![]()
Discussion of results
![]() Tin
chloride dissolves in water very well, but it tends to hydrolyse strongly. This
explains the turbidity of non-acidic tin (II) solutions.
Tin
chloride dissolves in water very well, but it tends to hydrolyse strongly. This
explains the turbidity of non-acidic tin (II) solutions.
![]() When the
solution of potassium iodide is added, then a yellow precipitate of tin (II)
iodide is formed.
When the
solution of potassium iodide is added, then a yellow precipitate of tin (II)
iodide is formed.
Sn2+(aq) + 2I–(aq) → SnI2(s)
When tin (II) chloride is added at high concentration then this precipitate turns orange. No explanation for this effect can be given. This is an interesting point of research and probably this effect and its explanation is known already somewhere.
![]() On addition of hydrochloric acid, the precipitate redissolves
again. This is because of formation of a chloro-complex of tin, the tetrachloro
stannate (II) ion.
On addition of hydrochloric acid, the precipitate redissolves
again. This is because of formation of a chloro-complex of tin, the tetrachloro
stannate (II) ion.
SnI2(s) + 4Cl–(aq) → SnCl42-(aq) + 2I–(aq)