


Allotropes of selenium
This experiment nicely shows how the vitreous gray/black form of selenium can be transformed into a red allotrope. The commercial form of selenium almost always is the grey/black form. It can be purchased in the form of a powder, or in the form of shot, having a shape like red corpuscles. In this experiment the shot form is used, for reasons of safety.
![]() Selenium, although it is an essential human nutrient, is very toxic at higher
doses. In this experiment, a minute amount of selenium is used in shot form, so
the risk is not that severe, but it is best to assure that no liquid is touched
and no fumes are inhaled.
Selenium, although it is an essential human nutrient, is very toxic at higher
doses. In this experiment, a minute amount of selenium is used in shot form, so
the risk is not that severe, but it is best to assure that no liquid is touched
and no fumes are inhaled.
![]()
![]() Required
chemicals:
Required
chemicals:
-
selenium, shot form
-
moderately concentrated nitric acid (50+ % HNO3)
-
potassium metabisulfite (sodium sulfite also works)
-
zinc
![]() Required
equipment:
Required
equipment:
-
test tubes
-
bunsen burner or equivalent for heating the contents of test tubes
![]() Safety:
Safety:
- Nitric acid is very corrosive, especially in the concentrated form, as required for this experiment.
- Selenium is toxic, although the shot form is not really risky.
- In this experiment, a small amount of selenium dioxide/selenous acid is formed. This is very toxic, so be careful not to touch any of the liquids. Also after dilution with water, when the concentration of the nitric acid is not very high anymore, still be careful because of the selenium contents.
- In this experiment some nitrogen dioxide is formed. This gas is insidiously toxic and its adverse effects can be delayed with several hours. Be sure that this gas is not inhaled. If in doubt, perform the first part of the experiment outside!
- The risk of this experiment is strongly reduced, because of the micro-quantities used for this experiment.
![]() Disposal:
Disposal:
- The amount of selenium, used in this experiment is so low (approximately 10 mg), that with a lot of water, the waste may be flushed down the drain.
![]()
Dissolving selenium in nitric acid
In this stage of the experiment, the selenium is dissolved in nitric acid. It is oxidized to selenium dioxide and converted to selenous acid in the strongly acidic environment.
![]() Take a single corpuscle of selenium with a diameter of 1.5 to
2 mm. Such a small corpuscle only contains approximately 10 mg of selenium. In
the picture below, the single corpuscle is shown, put in a test tube. The
picture is taken, such that the top and bottom border are precisely over the
wall of the test tube, so the height of the picture corresponds with the width
of a standard test tube. This gives an impression of the small size of the
corpuscle.
Take a single corpuscle of selenium with a diameter of 1.5 to
2 mm. Such a small corpuscle only contains approximately 10 mg of selenium. In
the picture below, the single corpuscle is shown, put in a test tube. The
picture is taken, such that the top and bottom border are precisely over the
wall of the test tube, so the height of the picture corresponds with the width
of a standard test tube. This gives an impression of the small size of the
corpuscle.

![]() Add
approximately 1 ml of nitric acid. When this is done, then no visible reaction
occurs. In order to dissolve the selenium, the acid should be heated somewhat.
Vigorous boiling certainly is not necessary. When the acid is hot, then the
corpuscle starts 'dancing' up and down through the liquid. It jumps up, when a
large bubble of gas is attached to it, and it falls down, when the bubble goes
to the surface. In this way, the corpuscle dissolves fairly quickly. The gas
mixture above the liquid becomes brown, due to formation of nitrogen dioxide,
and the liquid becomes yellow/green, due to dissolving of nitrous vapors in the
liquid.
Add
approximately 1 ml of nitric acid. When this is done, then no visible reaction
occurs. In order to dissolve the selenium, the acid should be heated somewhat.
Vigorous boiling certainly is not necessary. When the acid is hot, then the
corpuscle starts 'dancing' up and down through the liquid. It jumps up, when a
large bubble of gas is attached to it, and it falls down, when the bubble goes
to the surface. In this way, the corpuscle dissolves fairly quickly. The gas
mixture above the liquid becomes brown, due to formation of nitrogen dioxide,
and the liquid becomes yellow/green, due to dissolving of nitrous vapors in the
liquid.


![]() Continue
with this, until almost all of the corpuscle has dissolved. A tiny spot
should remain, this is needed for the second part of the experiment. When only a
small spot of the piece of selenium remains, then dilute the liquid, by adding
10 ml of water. Shake a little in order to dissolve all the nitric acid. After
this, a colorless solution is left, with a small dark grey disk of selenium in
it.
Continue
with this, until almost all of the corpuscle has dissolved. A tiny spot
should remain, this is needed for the second part of the experiment. When only a
small spot of the piece of selenium remains, then dilute the liquid, by adding
10 ml of water. Shake a little in order to dissolve all the nitric acid. After
this, a colorless solution is left, with a small dark grey disk of selenium in
it.
![]()
Precipitating selenium in the form of its red allotrope
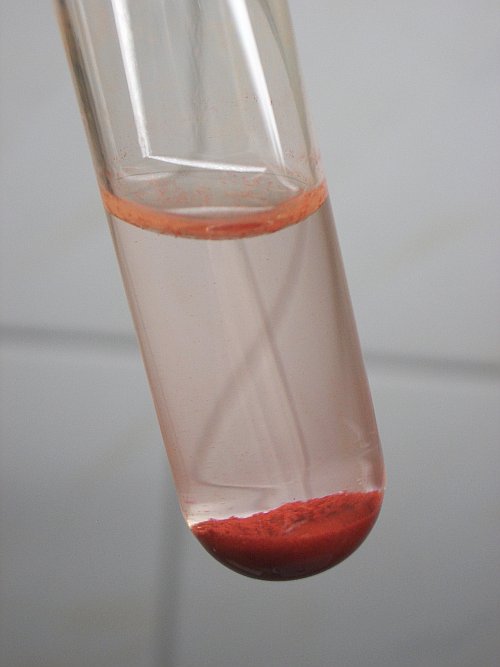
From the colorless liquid, obtained by dissolving the selenium, take approximately ⅔ part, without the piece of solid selenium. To this liquid add some solid sodium sulfite or potassium metabisulfite. Shake in order to dissolve the sulfite. This yields a colorless liquid, which slowly turns yellow, then orange and then it becomes turbid and a fine precipitate of selenium forms throughout the liquid. The color of the red allotrope of selenium really is bright. It is a nice deep red compound. The following sequence of pictures shows what is happening in a time frame of approximately 10 minutes, two to three minutes of time elapsed between two pictures.





The liquid with the red precipitate is allowed to stand for a few hours. The precipitate settles at the bottom and a beautiful bright red solid is formed!
The sample of selenium is kept for a while. and below, the sample is shown at different times:
- Immediately after preparation
- 17 days after preparation: the difference is not large


Keeping the sample of red selenium shows how stable this allotrope of selenium is.
![]()
Selenium-plated zinc
Now take the other ⅓ of the colorless liquid, together with the small disk of solid selenium. To this add some zinc. One would expect that the zinc dissolves fairly quickly in the still quite acidic environment with approximately 5% of nitric acid. Instead, the zinc becomes covered by a reddish layer of selenium. The initial bubbling of hydrogen gas quickly comes to a halt and the zinc becomes covered by selenium. The following two pictures show the pieces of zinc, covered with selenium, together with the remains of the original grey/black corpuscle of selenium. These pictures nicely demonstrate the strong contrast between the two allotropes of selenium.


In the left picture, the zinc and selenium are still under the liquid, with the test tube almost kept horizontal. In the right picture, the liquid was decanted from the test tube, with the zinc and selenium sticking at the glass wall. The test tube was kept upside down, in order to allow the picture to be made.
![]()
Discussion of results
Selenium can be oxidized by nitric acid fairly easily. The reaction equations are given below. Both reactions are occurring simultaneously and the exact ratio depends on temperature and concentration of the acid:
4HNO3 + Se → SeO2 + 4NO2 + 2H2O
4HNO3 + 3Se → 3SeO2 + 4NO + 2H2O
The selenium dioxide reacts with water and forms selenous acid. This acid remains almost completely undissociated in the strongly acidic environment.
SeO2 + H2O → H2SeO3
When the nitric acid is diluted approximately 10 times, then its oxidizing power is lost, it just is acidic. When sulfite (or metabisulfite) is added to the acidic solution, then sulphur dioxide is formed. This sulphur dioxide in turn reduces the selenium dioxide to the element.
Formation of sulphur dioxide: SO32– + 2H+ → SO2 + H2O
Reduction of selenium dioxide: 2SO2 + SeO2 + 2H2O → 2SO42– + 4H+ + Se
The selenium precipitates as a red solid.
With zinc metal a similar reaction occurs. The zinc reduces the selenium dioxide to selenium.
2Zn + SeO2 + 4H+ → 2Zn2+ + Se + 2H2O