


Rhenium chemistry
This set of experiments is added here, not because of the fact that this is very spectacular or exciting, but it is quite unusual. I was lucky to get my hands on a few grams of finely powdered rhenium for not too much money.
In this set of experiment, some properties of the compounds of rhenium in lower oxidation states are shown. Unfortunately, no real explanations can be given here, just observations. The chemistry of rhenium in lower oxidation states is poorly understood and a lot of research needs to be done in this field. The reason that this chemistry is poorly developed is that rhenium is exceedingly rare. It is one of the rarest elements.
![]()
![]() Required
chemicals:
Required
chemicals:
-
rhenium
-
nitric acid
-
hydrochloric acid
-
zinc
-
tin (II) chloride
-
sodium ascorbate or ascorbic acid
-
sodium hydroxide
-
potassium metabisulfite
-
sodium borohydride
![]() Required
equipment:
Required
equipment:
-
test tubes
![]() Safety:
Safety:
- Concentrated nitric acid and hydrochloric acid are corrosive. Especially nitric acid is quite destructive to the skin. If a splash of acid comes on the skin, immediately rinse with a lot of water.
- Sodium hydroxide is very caustic. If some of this comes on the skin, then rinse with water until the greasy feeling has disappeared.
- A small amount of nitrogen dioxide is formed in this experiment. This amount probably is so small, that no severe risks are imposed, but to be safe, the first step of dissolving the rhenium metal can best be done outside, or at least in a well-ventilated room.
- Sodium borohydride decomposes violently on contact with acids, giving a lot of hydrogen gas.
![]() Disposal:
Disposal:
- The amount of metal waste, generated in this experiment is so small, and the toxicity of the involved metals is so low, that it can be flushed down the drain with a lot of water.
![]()
Dissolving rhenium in nitric acid (step 1)
Rhenium metal can easily be dissolved in nitric acid. For this purpose, take approximately 25 mg of rhenium powder and add a few large drops of nitric acid (52% HNO3 by weight) to this. The two pictures show the small amount of rhenium metal in a test tube.


After an induction period of several seconds, the metal suddenly dissolves, with the evolution of brown nitrogen dioxide. Within a few minutes and with the help of a little heating, all of the metal is dissolved and a very light yellow liquid is obtained. The three pictures below show the dissolving of the metal in the concentrated nitric acid. In the left picture and also in the middle picture, one can clearly see a green color in the liquid. This green color is common, when a compound is dissolved in nitric acid. It is due to dissolved nitrogen oxides. Probably the color is due to mixing of blue N2O3 and brown NO2. After dissolving the metal a light yellow liquid is obtained, with some brown NO2 above it. The yellow compound still is due to some nitrous compound.



It is known that rhenium, when it is dissolved in nitric acid, goes into solution as perrhenic acid, HReO4. Perrhenic acid is a strong acid and hence, in solution there are ions ReO4–. The perrhenate ion is colorless.
After the metal is dissolved in the nitric acid, the test
tube is heated gently
 and the nitrogen dioxide is carefully blown away. The
liquid is not boiled. After this treatment, the liquid is diluted with
concentrated hydrochloric acid (30% HCl by weight). The final liquid still is
not completely colorless. The faint yellow color probably is due to the presence
of nitrosyl chloride, dissolved in the concentrated acid. The liquid is not pure
hydrochloric acid, but it also contains quite some nitric acid.
and the nitrogen dioxide is carefully blown away. The
liquid is not boiled. After this treatment, the liquid is diluted with
concentrated hydrochloric acid (30% HCl by weight). The final liquid still is
not completely colorless. The faint yellow color probably is due to the presence
of nitrosyl chloride, dissolved in the concentrated acid. The liquid is not pure
hydrochloric acid, but it also contains quite some nitric acid.
When nitric acid and hydrochloric acid are mixed and stored for a while, then the liquid also becomes yellow, due to formation of nitrosyl chloride and also some chlorine.
The almost colorless liquid, as it is obtained here, is used as a starting point for further experiments with rhenium.
The spectral absorption properties of perrhenic ion are similar to those of permanganate. Both ions show a strong absorption and hence have intense 'color'. For permanganate the absorption band is in the visible region, giving it its intense purple color. For perrhenate, the absorption band is in the UV-range. So, for the human eye, perrhenate is colorless.
The perrhenate ion only is a mild oxidizer. This is in strong contrast with the corresponding permanganate ion.
![]()
Reduction of the perrhenate with zinc
The perrhenate, dissolved in hydrochloric acid, can be reduced with zinc metal. This reduction is not very good, because of the fact that most of the zinc dissolves in the acid, forming hydrogen gas and zinc (II) ions. Some of the zinc, however, reduces the perrhenate ion. This is shown here. The result of the reaction strongly depends on the concentration of the acid.
![]()
Reduction of perrhenate with tin (II) chloride
When the perrhenate is reduced with tin (II) chloride, then the result strongly depends on the amount of nitric acid, left in the liquid. The tin (II) chloride reacts with nitric acid, with the formation of a colorless gas (probably NO) and the production of quite some heat. Before the tin (II) chloride is used up by excess nitric acid, it is capable of reducing the rhenium compound to a bluish/green compound. Two experiments, with different experimental conditions are given here.
![]()
Reduction of perrhenate with sulphur dioxide and borohydride
In this experiment it is shown that perrhenate is not reduced by sulfite. When a borohydride is added, then the perrhenate is brought to reaction. The sulfide, formed from the sulfite, reacts with the perrhenate and a black solid compound is formed. Is this Re2S7 or ReS2? A more complete description of this experiment is given here.
![]()
Hydrolysis on dilution and removal of acid
The yellow/green compound, formed on reduction of perrhenate with zinc metal, is stable in strong hydrochloric acid, but when the concentration of the acid is reduced, then it can be converted to a brown compound. In order to bring about the conversion, heat is required, however.
The yellow/green liquid first is diluted to three times its original volume. This results in a light yellow liquid.
In a separate test tube, a solution of sodium hydroxide is made by adding a spatula full of the solid to a small amount of water and some of this solution is added to the light yellow/green solution. One must assure that excess acid remains in solution. When some solution of sodium hydroxide is added, then a white precipitate is formed, which dissolves again on shaking. If this precipitate does not dissolve anymore, then too much sodium hydroxide is added. Adding a few drops of dilute hydrochloric acid then can restore the acidity of the liquid and make it clear again.
Finally, this results in a light yellow liquid, which just is slightly acidic.
The color of the yellow/green liquid does not change very much, when the solution of sodium hydroxide is added. It becomes lighter, due to dilution, and possibly a little more yellow. When the liquid is boiled gently for a few minutes, then the solution changes color to a light reddish brown.
The two pictures below show the liquid with the sodium hydroxide added, before heating and the same liquid after boiling it for a few minutes. After boiling, the liquid has turned brown.

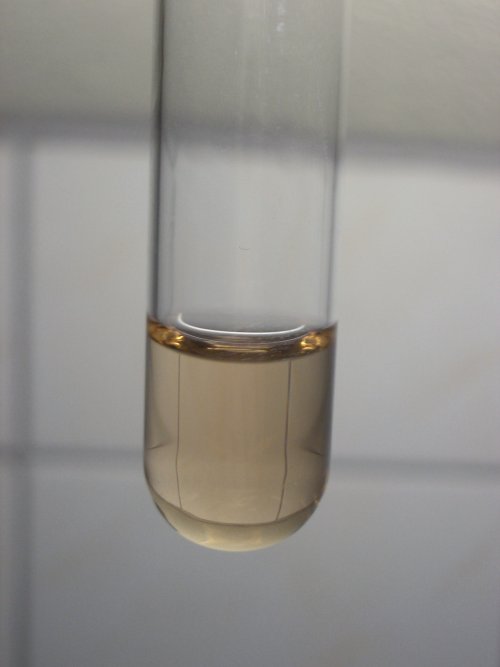
The change of color probably is due to hydrolysis of the green compound. The tinge of the color resembles that of the product, which is obtained after reduction with excess tin (II) chloride. The exact composition of the brown compound in the liquid, however, is not clear.
![]()
Discussion of results
When rhenium is dissolved in concentrated nitric acid, then the following reactions occur:
Re + 7HNO3 → 7NO2 + HReO4 + H2O
The compound HReO4 is called perrhenic acid. This is a colorless compound, which is a fairly strong acid. So, it may split into the ions H+ and the perrhenate ion ReO4–.
The gas NO2 can be observed as the brown gas above the liquid. It also dissolves in the liquid, where it reacts with water, forming back nitric acid and NO. This mix in the nitric acid causes the green color of the liquid (NO and NO2 form blue N2O3, which gives with brown dissolved NO2 a green color).
When the liquid is diluted, hydrochloric acid is added and zinc is added, then the perrhenate ion is reduced. In the presence of a lot of chloride ions, the hexachloro-rhenate (IV) ion is formed easily.
3Zn + 12Cl– + 2ReO4– + 16H+ → 3Zn2+ + 2ReCl62- + 8H2O
In literature, the ion ReCl62- is said to be green. When the pH increases, then the ion ReCl62- tends to hydrolyse, forming oxo-Re-species, finally forming ReO2, which according to literature is black/brown. The brown color in the last experiment may be such an oxo-species of rhenium in its +4 oxidation state.
Rhenium can form remarkable compounds at even lower oxidation state. Possibly, such a compound is formed when excess tin (II) chloride is used as reductor, but chances are better that these are not formed under the experimental conditions of these experiments. In literature a bright blue chloro-rhenium complex is described with rhenium in its +3 oxidation state, but probably this can only be prepared with rigorous exclusion of air.