


Color of red cabbage juice as function of pH
Red cabbage for many people is a nice vegetable, which can be part of a good dish, but it also is a nice ingredient of a chemistry experiment, which even can be carried out by not too young children.

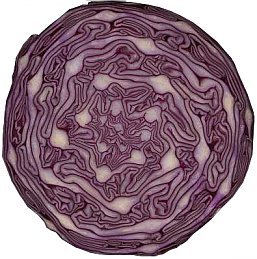
It is well known that red cabbage can have any color ranging from red/purple to blue/purple. At low pH it appears red, at higher pH it appears blue/purple.
Much less known, however, is that it can be intensely bright red, green or golden yellow. These colors are obtained at pH values, which normally are not applied in the kitchen. This very simple experiment nicely demonstrates that red cabbage juice can be used as a crude pH indicator, with many different colors.
![]()
![]() Required
chemicals:
Required
chemicals:
-
red cabbage juice, prepared by boiling some red cabbage in water
-
dilute hydrochloric acid
-
vinegar
-
dish washing soap
-
ammonia
-
sodium carbonate (or sodium hydroxide)
![]() Required
equipment:
Required
equipment:
-
test tubes
![]() Safety:
Safety:
- This is a typical experiment, which can be carried out by somewhat older children, but if that is done, then still good supervision is needed and in that case, the solutions of hydrochloric acid and ammonia must be kept dilute (less than 3%).
- Sodium hydroxide is very corrosive, sodium carbonate can also be used and is a safer alternative.
![]() Disposal:
Disposal:
- The waste can be flushed down the drain.
![]()
Preparation of red cabbage juice
Cut some red cabbage in small parts and boil for several minutes in a small volume of water. Take 100 ml of water and 50 gram of red cabbage. This gives sufficient juice of good concentration for many experiments. Decant the juice from the solid vegetable and let it cool down.
Take 5 test tubes, and in each of the test tubes, pour a few ml of the red cabbage juice. Then, add the following reagents to the test tubes (one reagent per test tube, 1 to 2 ml per reagent):
- dilute hydrochloric acid
- vinegar
- dish washing soap
- dilute ammonia
- concentrated sodium carbonate (or dilute sodium hydroxide)
The picture below shows all 5 test tubes:

From left to right, these are test tubes with reagents (1) to (5) added as mentioned above. So, at very low pH, red cabbage juice can have a deep bright red color, and at very high pH, the red cabbage juice is golden yellow.
With such color variations, depending on pH, red cabbage can nicely be used as a crude pH indicator. The color of the solution depends on pH as follows:
| pH | color |
| 1 | bright red |
| 4 | pink/rose |
| 8 | purple/blue |
| 10 | green |
| 12 | golden yellow |
![]()
Discussion of results
Red cabbage contains two types of water-soluble pigments, whose color depends on the pH of the solution:
- anthocyanins, which are red at low pH, blue at high pH and colorless at very high pH
- anthoxanthins, which are colorless at low pH and yellow at high pH.
The presence of these two pigments nicely demonstrates the colors, which can be obtained from the juice of red cabbage:
- at pH = 0, the anthocyanin is red, the anthoxanthin is colorless
- at pH = 4, the anthocyanin is still red, but a little more towards purple already, the anthoxanthin still is colorless
- at pH = 8, the anthocyanin is blue, and the anthoxanthin still is colorless
- at pH = 10, the anthocyanin still is blue, the anthoxanthin now is yellow, giving a combined green color
- at pH = 12, the anthocyanin is colorless, the anthoxanthin still is yellow.
Anthocyanins, structure depending on pH
The anthocyanins have a structure as shown below, it is a whole class of compounds, derived from a chemical entity, called cyanidin.
At low pH, these form cationic species, with a positive charge, concentrated in the ring with the oxygen atom, which is marked with a +.
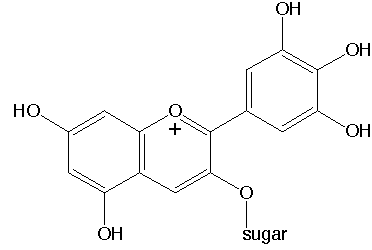
At higher pH, the top right aromatic ring forms a quinone-like structure, and a negative charge flows from the middle -OH group of that structure to the positively charged ring. This change in electronic structure is accompanied with a change in color (from red to blue).
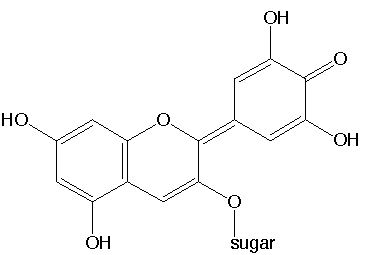
At even higher pH, the top left -OH structure at the left aromatic ring can loose a proton, and a shift of charge occurs through the molecule, where the left aromatic ring also forms a quinone-like structure and the right ring becomes aromatic in nature again and a negative charge builds on the middle oxygen of the right ring. Equally well, a situation could exist, where the left ring remains aromatic (just looses a proton from its top-left -OH structure) and the right ring has quinone-like structure, with a negative charge on the top-left oxygen atom. In reality there will be a resonance structure, extending over the entire anionic species at such high pH. This species is colorless, but given the large size of the resonance structure, it most likely will have strong absorption in the infrared range of the spectrum (this last thing, however, is speculation of the author of this website and has no hard scientific backup).
Anthoxanthins, structure depending on pH
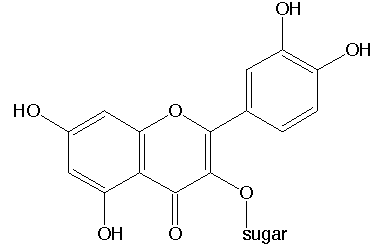
White (colorless) anthoxanthins are compounds, derived from a chemical compound, called quercetin. The structure of molecules in this class of compounds strongly resembles the structure of the anthocyanins.
At low pH, these form neutral molecules with a structure, as given below.
At high pH, a resonance structure is formed, in which one of the -OH groups looses a proton (either at the top-left or at the middle right) and a negatively charged structure is formed, which forms a resonance with the =O at the bottom middle. The color of such a resonant structure is deep yelllow.
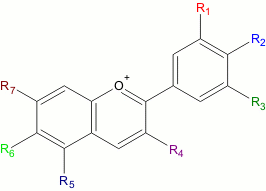
Remark 1: The explanation, given above, is a simplification of reality. The class of anthocyanins and anthoxanthins is broader. Many anthocyanins and anthoxanthins exist (more than 500 are known nowadays) and these can be imagined as being similar to the structures, shown above, but with one or more of the -OH groups being replaced by other groups (e.g. -H or -OCH3). Below, the general structure of the class of anthocyanins at low pH is shown. A similar freedom exists for anthoxanthins.
Remark 2: More information is available in the document, given below. It describes the chemistry of the colors of flowers in an easy to understand way:
http://www.genetics.org/cgi/reprint/32/4/410.pdf