


Thionyl chloride and potassium dichromate
Dichromate ion is a strong oxidizer in acidic environments and it is very easily reduced. It is well-known that sulphur dioxide, which contains sulphur in the +4 oxidation state, reduces dichromate ion at once, and green chromium(III) is formed. Remarkably, in thionyl chloride, which also contains sulphur in the +4, dichromate ion is not reduced, not even after a full day of storage of the mix.
Potassium dichromate dissolves reasonably well in thionyl chloride, giving a bright red solution, and a faint orange vapor is formed above the liquid. This almost certainly is due to formation of chromyl chloride. Most likely the reaction between potassium dichromate and thionyl chloride is as follows:
K2Cr2O7 + SOCl2 → 2KCl + 2CrO3 + SO2
CrO3 + SOCl2 → CrO2Cl2 + SO2
When concentrated (96%) sulphuric acid is added, then the potassium dichromate dissolves more easily, and a deep red (in thicker layers almost black) liquid is formed. This deep red liquid slowly gives off HCl, it is bubbling a little bit like coca cola. When this liquid is kept in a closed bottle, then there is slow pressure buildup. If it is loosely capped, then fumes of HCl are released from the bottle slowly, at a fairly constant rate. The following picture shows this bottle, with the deep red liquid and the thin vapor of chromyl chloride above it.
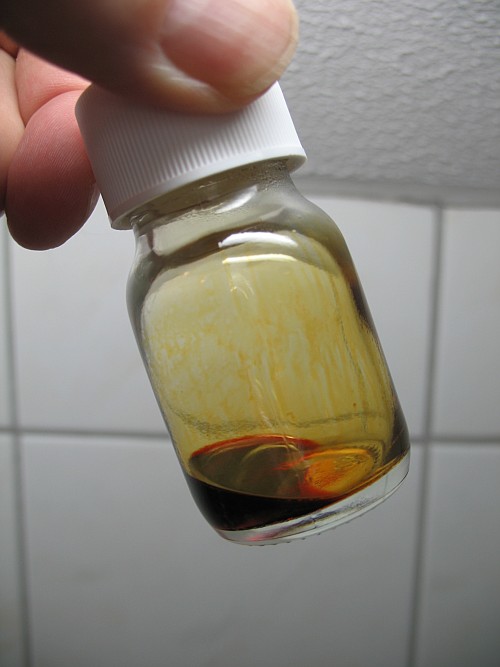
For this experiment, approximately 50 mg of powdered potassium dichromate was added to around 0.5 ml of thionyl chloride. To this, approximately 1.5 ml of concentrated sulphuric acid was added. This mix (after dissolving all solid potassium dichromate) is shown in the picture above.
![]()
Mixing the H2SO4/SOCl2/K2Cr2O7 mix with water
In the mix, no reduction of chromium(VI) occurs, even although a lot of sulphur in oxidation state +4 is present at high concentration. When the liquid is added to water, then the situation changes drastically. The hexavalent chromium is converted to green chromium(III) at once. There also is a very violent reaction with water, giving copious amounts of gas (HCl and SO2).
When the liquid is added to water, then also much more chromyl chloride is formed. The fumes/gasses, formed in the reaction with water, have a much stronger color, than the color of the vapor above the deep red liquid.
The picture below shows what happens when this liquid is added to water.

The reaction with the water is very violent. A lot of brown/orange gas is formed, and the water is almost swirled out of the erlenmeyer. Every drop of liquid, which falls in the water gives a hissing noise. The ten pictures below show a total time frame of approximately 300 ms.










One second later, a lot of orange vapor was around the erlenmeyer. In the liquid, there are many small droplets of the dark red liquid. These little droplets dissolve in the water very quickly and the color then becomes green.

Just a few seconds after that, the air around the erlenmeyer was totally filled with a dense off-white fume of humid HCl, contaminated with some chromyl chloride. Only 30 seconds later, the fumes were gone, and a clear bright green liquid remained. Pictures of both situations are shown below.


When the liquid is mixed with water, then any remaining thionyl chloride reacts with water, giving HCl and SO2. The HCl causes formation of dense fumes around the erlenmeyer. The hexavalent chromium is reduced by the SO2, formed in the reaction with the water. At once, green chromium(III) appears.
The video of the experiment can be downloaded, by clicking the link below. The sound unfortunately is not really good, there is a lot of noise. Because of the copious amounts of toxic fumes, formed in this experiment, the fan of the fume hood was running at max power. This is the noise in the video. Download size of the video is approximately 3.3 MByte.
Video of violent reaction with water: violent.avi.