


Antimony trisulfide, from grey to yellow
Antimony trisulfide is a chemical, used in matches, and also in fireworks displays. In this experiment, however, it is not used for pyrotechnic purposes.
Here, it is used to show how different a chemical can be, when it is precipitated from water, compared to the dry chemical. The dry chemical is a near black solid. In finely powdered form it is dark grey.

![]()
In this experiment, some of the dry solid is added to concentrated hydrochloric acid and the liquid then is gently heated to 60 ºC or so. This results in slow dissolving of the solid. An almost colorless liquid is obtained, and also some colorless gas is produced. The colorless gas is hydrogen sulfide, which has a very strong smell of rotten eggs. Beware: this gas is very toxic, and this experiment must be done in a really well ventilated room, or even better, it must be done outside or in a fume hood. High concentrations of hydrogen sulfide can deaden your sense of smell and give you a false sense of safety, because you don't smell the gas anymore.
The picture below shows some of the dry solid in a test tube.

To this solid, approximately 2 ml of concentrated hydrochloric acid is added. After some heating, one clearly sees small bubbles of hydrogen sulfide and the solid slowly disappears.

The equation, describing this reaction, can best be given as follows:
Sb2S3(s) + 6H+ + 6Cl– → 2SbCl3(aq) + 3H2S(aq, g)
With the notation (aq, g) it is meant that the hydrogen sulfide appears both as gas and dissolved in the liquid. The compound SbCl3 only can exist as such in very concentrated hydrochloric acid. When the acid is diluted, it immediately hydrolyses.
When most of the solid has dissolved, then the solution at once is poured in a large excess of water. This results in formation of a bright yellow/ochre precipitate. This yellow precipitate is hydrous Sb2S3.
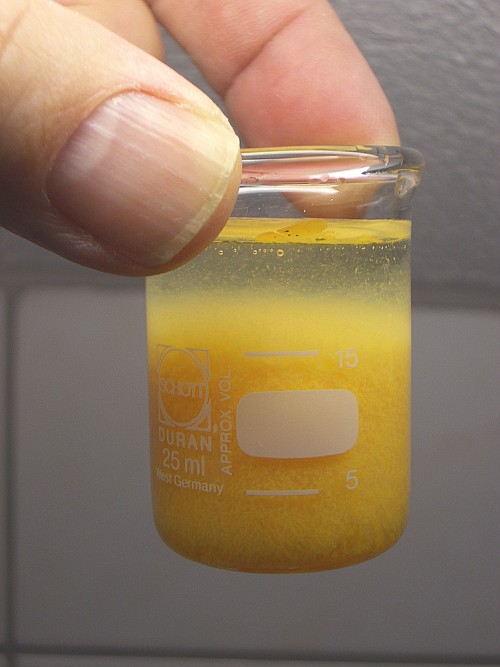
On dilution, the SbCl3 at once hydrolyses and a reaction occurs with the H2S, present in solution:
2SbCl3(aq) + 3H2S(aq) + nH2O → Sb2S3·nH2O(s) + 6H+ + 6Cl–
The reaction in fact is very close to the reverse of the reaction, where the solid grey Sb2S3 dissolves in the concentrated acid. The main difference is that the Sb2S3, formed in this reaction is a hydrous form. This hydrous form is totally different from the dry form. If the yellow solid is dried and heated, then it looses water and reverts back to the black form.
The picture below shows what happens if the liquid with the yellow precipitate is poured on a ceramic surface. One can nicely see the coarse yellow solid.

It really is remarkable to see how an anhydrous compound and a hydrous compound can differ so much. This phenomenon is quite well known and is most pronounced for the sulfides of the elements Sb and As. One can safely state that Sb2S3·nH2O(s) has a totally different structure than Sb2S3, otherwise there would not be such a large difference in its properties.