


Collapse of polystyrene packaging material
A really funny experiment is the collapsing of polystyrene packaging material. Polystyrene is a polymer of styrene. Its formation is best described by means of the following drawing:
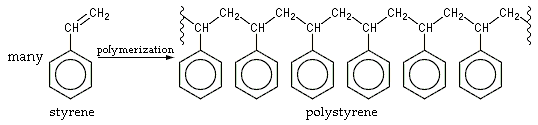
Polystyrene is a colorless/transparent plastic, used for cheap one-use drinking glasses, beakers, etc. This material, however, also can be made into a very voluminous low-density foam, which is used extensively as packaging material.

This foam usually is called "expanded polystyrene', also known as styrofoam. This is made by a mix of polystyrene and some gaseous blowing agent. The gaseous blowing agent can be any non-reactive gas, like air, or CO2. After solidification of the polystyrene, a structurally stable and very light product remains. It has good strength, while having very low density, and that makes this expanded polystyrene ideal for packaging purposes. Due to the enclosed gas in the foam, it also has good heat insulating properties and it also is applied for that purpose. This material has its properties, because the chains of polystyrene molecules have cross-links as well, giving it good structural stability.
When acetone is added to this expanded polystyrene, then it collapses. The polystyrene strands "dissolve" in the acetone, removing the cross-links with other strands of polystyrene. This results in loss of structural stability, and the enclosed gas in the foam simply bubbles out of the material, and the material itself collapses. This is a funny demonstration, which only requires very simple chemicals. The picture below shows a piece of styrofoam, which is severely damaged by acetone.

Performing the experiment is easy. Take approximately 15 ml of acetone and a piece of styrofoam of 50 cm length. Pour the acetone over the styrofoam and watch the material collapse.

The following video nicely shows this experiment: collapsing styrofoam. Download size is approximately 4 MByte.