|
Making nitric acid 90% With an all-glass distillation setup, one can nicely concentrate commercially available 60% to 70% nitric acid to a concentration of 90% or even better. No vacuum distillation is needed for that, the only disadvantage is that the resulting acid contains some nitrogen dioxide. This nitrogen dioxide is formed during distillation, due to slight decomposition of the nitric acid. In this experiment, the following mix was prepared:
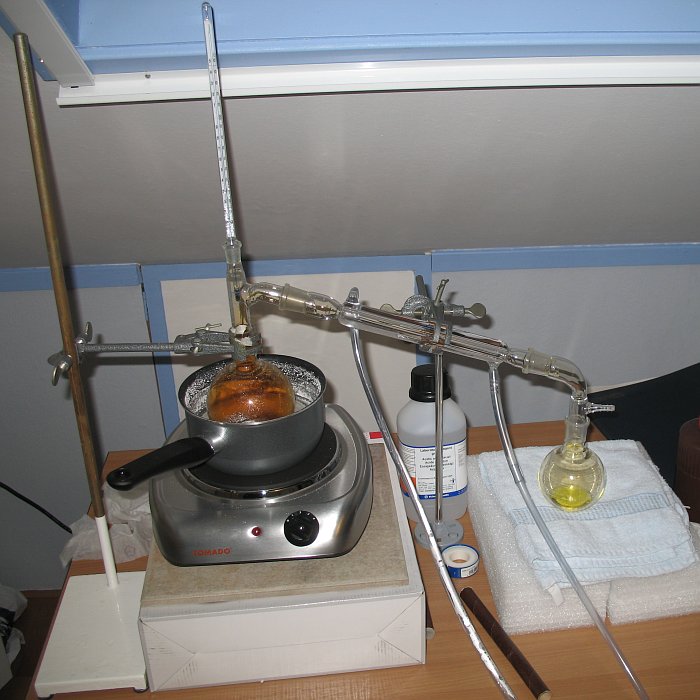
The sulphuric acid is added carefully and slowly to the nitric acid. After mixing, some white fumes can be observed already. This mix is put in a round bottom flask, and this is immersed in a bath of concentrated calcium chloride solution. This bath then is heated on a hot plate. No oil bath is used, due to the high risk of explosion when the flask breaks. Hot nitric acid/sulphuric acid mix violently oxidizes the oil of the oil bath. With calcium chloride mix, such reaction does not occur. The disadvantage of this calcium chloride mix is that the water in this mix quickly evaporates and a lot of steam is produced during the distillation, which is inconvenient. I did the distillation below a roof-window, which was open all the time (cold in wintertime, but at least the house does not get humid). Another disadvantage of the use of the calcium chloride bath is that it does not get really hot. The heat was just sufficient for distilling the nitric acid, but distilling water with this setup is not possible. That extra 10 degrees cannot be achieved anymore and the water simply refluxes in the heated round bottom flask. In this setup, the last disadvantage was turned into an advantage. Distillation was continued, until nothing comes over anymore. When all nitric acid has come over, the heating source is not capable of making the water come over. So, the distillation was carried on, until dripping into the collector flask became very slow. The distillation setup looks as follows:
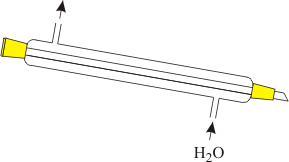
The vapor of the nitric acid is cooled/condensed in a liebig cooler, using slowly running tap water and drops of the condensed acid are collected in a smaller round bottom flask. The collector flask is not cooled anymore. A schematic diagram of the liebig cooler shows how it works. The inner tube contains the vapor to be condensed, the outer tube is filled with slowly running cold tap water.
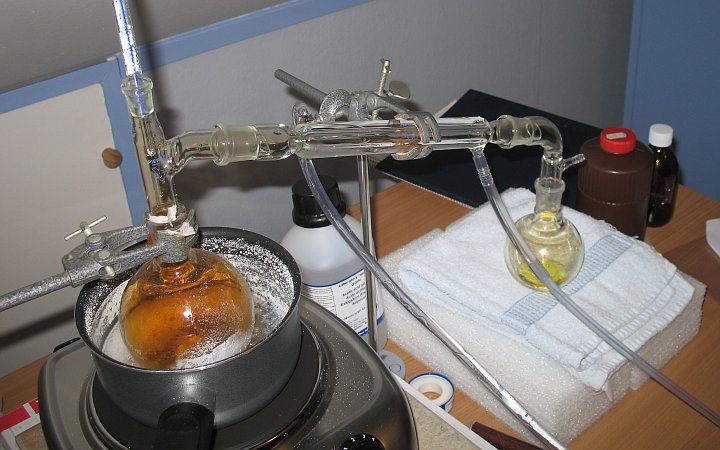
A close-up of the distillation setup is shown in the picture below:
The acid in the collector flask has a bright yellow color, and there is a faint color of nitrogen dioxide above the acid. The picture shows approximately 10 ml of acid.
The thermometer above the heated flask shows a vapor temperature of just over 90 ºC. Nitric acid, boiling at this temperature has a concentration well over 90%. The remaining impurity mainly is water, and some NO2. The heated flask has a brown atmosphere of NO2, but in the collector flask, the amount of NO2 already is much lower. If one wants really pure nitric acid, then this yellow product must be redistilled at lower pressure and the early boiling stuff then must be discarded (that contains most of the NO2).
The yellow nitric acid is amazingly corrosive. When it is dripped on table salt, or in concentrated hydrochloric acid, then a fairly vigorous reaction occurs, in which an orange gas mix is produced (nitrosyl chloride and chlorine). The yellow acid also is strongly fuming. The acid is collected in a small brown bottle of 10 ml, and when this is opened, then dense white fumes can be seen.
When one is looking carefully at the fume, then one sees that it consists of many small droplets. A close-up of this nicely shows this:
The fuming also is demonstrated in a small video (download size is approximately 2.8 MByte). The total yield is just over 10 ml of concentrated acid, and given the amount of starting material, this is quite good. |
|
|
|
|
|
|
|
|
|
|