


Green fire without pyrotechnics
In every day life, people are used to fire with orange flames. Other colors only are known from fireworks and from gas flames. This very simple experiment shows a simple fire (combustion of a solid). As with any other fire, oxygen is used from the air.
The material, used in the experiment is the chemical dimethylamine borane. This is a complex with the following structure:
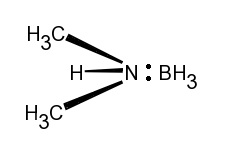
The lone pair of electrons on the nitrogen atom is shared with the boron atom in the borane groep, BH3.
This chemical is a volatile, and flammable chemical. It burns with a nice green flame, when ignited. This green flame color is very characteristic of burning boron compounds. Another experiment, with a similar flame color, using more easily obtained chemicals, is available here.
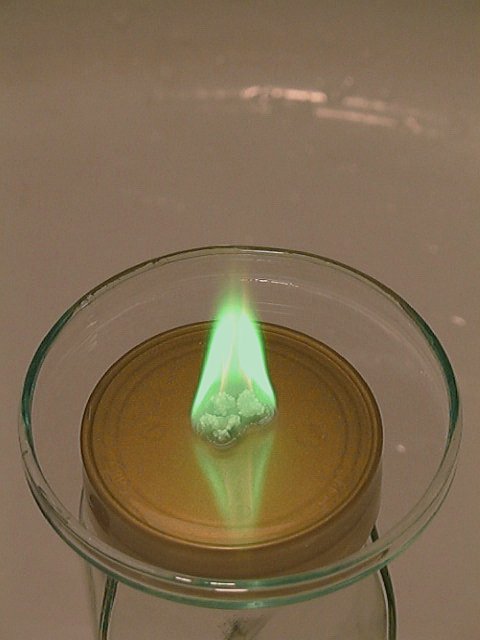
A small video is made of this burning compound: greenfire.avi. Download size is just over 1.5 MByte.