


Colored flames with metal salts
Many metal ions give a color to fire and flames. This phenomenon is used extensively in pyrotechnics, but it also can be used to make nice colored fire, without the need of pyrotechnics. Simply dissolving a suitable metal salt in alcohol and then burning a piece of paper tissue, soaked in the alcohol gives a colored flame.
 Normally,
fire is yellow/orange, as shown in the little picture at the left. This color is
caused by intermediate formation of small carbon particles, which starts glowing
in the heat of the fire. Most compounds give rise to formation of solid carbon
during combustion, hence the very familiar appearance of yellow/orange fire.
Normally,
fire is yellow/orange, as shown in the little picture at the left. This color is
caused by intermediate formation of small carbon particles, which starts glowing
in the heat of the fire. Most compounds give rise to formation of solid carbon
during combustion, hence the very familiar appearance of yellow/orange fire.
When no carbon particles are formed during combustion, then an almost invisible pale blue flame is created. This is the case when lower alcohols like methanol or ethanol are burnt. Such compounds, which hardly give a colored flame on their own, are very suitable for adding flame color by means of metal ions. Like carbon particles, many metal ions give color to a flame and this flame color is observed best, when no carbon particles are formed.
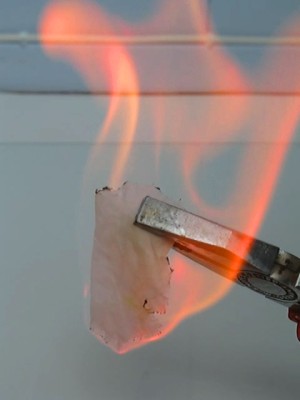
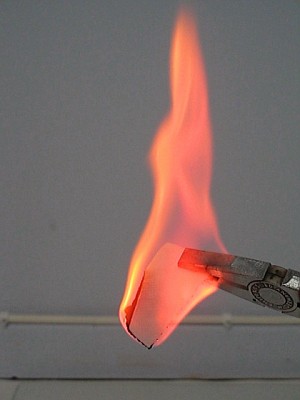
This experiment was done with with copper(II) chloride, strontium chloride, lithium chloride, and lithium bromide. The salts dissolve in lower alcohols. Copper(II) chloride dissolves exceptionally well, but strontium chloride also dissolves sufficiently well to give a nice effect. The lithium salts first must be dissolved in a single drop of water, and then alcohol needs to be added. It does not matter, whether hydrated salts or anhydrous salts are used for this experiment.
In fact, this is a very simple experiment. Dissolve the salt in some alcohol (ethanol or methanol can be used, in a concentration of 85% to 95%). Soak a piece of paper tissue in the alcohol and then light the piece of paper.
The result with copper chloride and strontium chloride is shown in the two upper pictures (left is copper chloride, right is strontium chloride). The result with lithium chloride and lithium bromide is shown in the lower pictures (left is lithium chloride, right is lithium bromide).


Videos can be downloaded of both experiments:
- burning of ethanol with copper(II) chloride (4.6 MByte)
- burning of ethanol with strontium chloride (3.2 MByte)
- burning of ethanol with lithium chloride (3.9 MByte)
- burning of methanol with lithium bromide (2.5 MByte)