


TCCA, Na-DCCA and BCDMH, nice experiments
This page contains a few experiments with trichloroisocyanuric acid (TCCA, swimming pool chlorine, slow release) and the related compound sodium dichloroisocyanurate (Na-DCCA, swimming pool chlorine, shock treatment). Below follow the structures of these two interesting chemicals, left is TCCA and right is Na-DCCA:
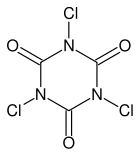
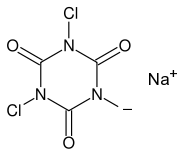
TCCA and Na-DCCA are very strong oxidizers, which also are very compact. For a given weight, these chemicals are remarkably powerful and they can produce lots of chlorine, when hydrochloric acid is added to them. They are interesting chemicals for a home lab and allow many experiments to be done, and they are easy to obtain. They are the modern variation of chlorine-based chemicals and more and more replace the classical sodium hypochlorite and calcium hypochlorite.
Another set of experiments is done with a related chemical, called BCDMH (1-bromo, 3-chloro, 5,5-dimethyl hydantoin). The structure of the BCDMH molecule is as follows:
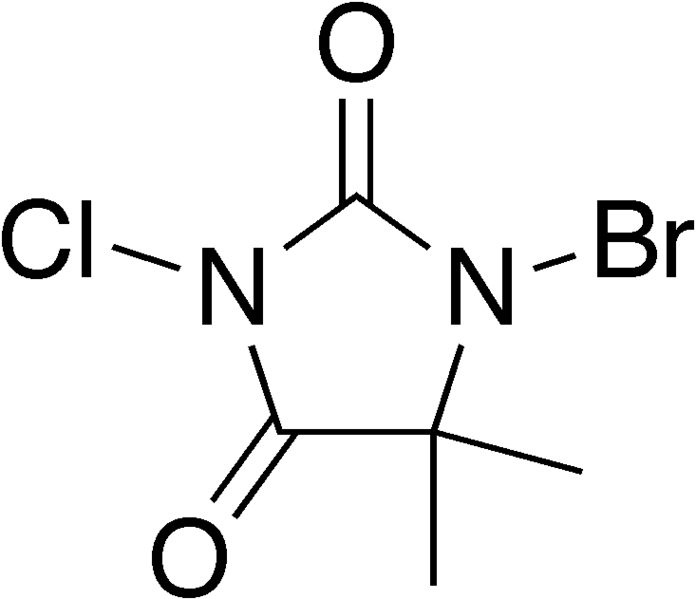
BCDMH also is a strong oxidizer, albeit not as strong as TCCA and Na-DCCA. This compound easily looses its bromine atom and to a somewhat lesser extent also its chlorine atom. BCDMH is sometimes used instead of TCCA, because some people find this less irritating to the eyes.
![]()
Copper (II) ions and dichloroisocyanurate ions
Copper(II) ion and the dichloroisocyanurate ion give rise to formation of a beautifully colored precipitate, which has a remarkable color for a copper-precipitate. Normally, copper(II) precipitates have colors, ranging from blue to green, sometimes with exceptions in the direction of black. This precipitate, however, has a really bright purple color!


The two pictures above show a precipitate made from a solution of copper sulfate and a solution of Na-DCCA. The left is a small, still somewhat wet amount. The right is a dried amount, close to 2 grams. The wet amount has many darker blue spots in a lighter surrounding. Probably the difference is due to particle size. When all is ground and dried, then the color difference disappears.
The precise nature of this purple precipitate is not known to me. Is this a copper(III) compound? It is known that copper (II) forms copper(III) compounds with strongly oxidizing compounds, even in aqueous solution. Maybe this is one of them. Anyway, the precipitate is quite remarkable for a copper compound.
![]()
Preparation of cyanuric acid
Below follow pictures of cyanuric acid, crystallized from a solution in water. The cyanuric acid was made by reacting Na-DCCA with dilute hydrochloric acid and driving off all chlorine by boiling the liquid. On cooling down the crystals are formed.


Cyanuric acid is formed by stripping off the chlorine atoms and replacing them with hydrogen atoms. This happens, when TCCA or Na-DCCA is allowed to react with an acid, while at the same time taking away HOCl, which is formed in replacing Cl-atoms by H-atoms from water. A solution of HCl does this job, by reducing the HOCl to chlorine.
Cyanuric acid exists in two tautomeric forms:
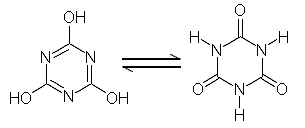
The left is the acidic form, in solution, both forms coexist and they readily convert into each other.
![]()
Funny reaction between ammonia and TCCA
When plain 5% household ammonia is added to solid TCCA, then the solid reacts vigorously with the ammonia and a lot of gas and smoke is produced. This reaction is quite impressive and it really is fun to see this happen. Below follow two pictures of a piece of TCCA, to which some ammonia is added.


A video of this experiment can also be downloaded: TCCA+NH3. Download size is approximately 3 MByte. Two other nice videos can be downloaded here:
- TCCA+NH3 - 2, download size is approximately 2 MByte
- TCCA+NH3 - 3, download size is approximately 4 MByte (video unfortunately has a hiccup, but otherwise it is quite stunning)
A very remarkable property of this reaction is that free chlorine gas is produced, if there is excess TCCA. Remarkable thing in this reaction is that free chlorine is produced in an alkaline environment with ammonia. With some logical reasoning, this, however, can be explained.
TCCA is a strong oxidizer, and it oxidizes ammonia to nitrogen gas, itself being converted to cyanuric acid, and hydrogen chloride being produced as well:
C3N3O3Cl3 + 2NH3 → C3N3O3H3 + N2 + 3HCl
The HCl in turn reacts with other surrounding ammonia, giving the thick smoke, the smoke being NH4Cl.
If only a little amount of ammonia is used, then the HCl also reacts with more TCCA, to give chlorine gas:
C3N3O3Cl3 + 3HCl → C3N3O3H3 + 3Cl2
Chlorine gas also reacts with ammonia, giving more nitrogen gas and ammonium chloride, but there also is a side reaction which gives NH2Cl, a colorless and very toxic gas, with a strong pungent smell. The smoke has a strong smell of NH2Cl. The visible part is from the non-toxic NH4Cl, but the smell and burning sensation in the eyes is from the small amounts of NH2Cl.
This theory of formation of chlorine from the TCCA and ammonia, when excess TCCA is used, is nicely confirmed, if some KBr is added to the TCCA. If that is done, and then some ammonia is added, then one still sees the bubbling of nitrogen gas, and the formation of thick white smoke, but there also is clearly visible production of bromine vapor. This only is visible if there is a real excess amount of TCCA, otherwise there is just vigorous bubbling and white smoke.
A video of this production of bromine can be downloaded: TCCA+KBr+NH3. Download size is just over 2.5 MByte.
![]()
Related reaction between ammonia and BCDMH
The chemical compound BCDMH (1-bromo, 3-chloro, 5,5-dimethyl hydantoin) also is used as a swimming pool chemical, but this chemical is used as brominating agent. The purpose of adding this chemical to the swimming pool water is very similar to the purpose of adding TCCA or a related chemical. Bromine-based chemicals are said to be somewhat less irritating to the eyes, but the use of bromine is more expensive, and in some countries it is not allowed for use in public swimming pools.
The compound BCDMH also reacts with ammonia, very much like TCCA, but the reaction is somewhat less violent, and if an excess amount of BCDMH is used, then a very interesting effect is created due to the formation of bromine vapor.
A mix of bromine vapor and ammonium bromide is formed and this looks very interesting. This mix also is very heavy and can be poured out of the beaker. The pictures below show the brown mix of bromine vapor and ammonium bromide smoke, the left picture just after formation, the right picture after part of the smoke is poured out of the beaker.


When excess ammonia is used, then the final color of the smoke is white, otherwise it is brown/orange. The following 5 videos show the reaction between BCDMH with different variations:
Videos made with a light background:
- excess ammonia (download size is 5.1 MByte)
- excess BCDMH (download size is 4.2 MByte)
- pouring out of the smoke (download size is 2.8 MByte)
Videos made with a dark background:
- excess ammonia (download size is 4.3 MByte)
- excess BCDMH (download size is 3.7 MByte)