


Formation and properties of polychromate (VI) salts
It is well known that hexavalent chromium forms chromates and dichromates. Chromates usually are yellow, and dichromates usually are bright orange.
Much less common are higher polychromates, the trichromates and tetrachromates. In this experiment, it is shown that the trichromates (and possibly tetrachromates) can be made fairly easily from the dichromates. Some differences with the dichromates are shown as well.
The property of forming polyanions is somewhat developed for chromium. Only the dichromates and to a lesser extent the trichromates are really stable. Tetrachromate is at the border of what can be achieved with chromium. Molybdenum and tungsten, the heavier congeners of chromium have a much stronger tendency of forming polyanions. These metals can form incredibly complicated anions, which may contain tens of metal atoms, with oxygen bridges between them.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium dichromate
-
ammonium dichromate
-
concentrated nitric acid
-
red phosphorus
-
sodium sulfite (for cleanup purposes)
-
potassium chromate (for display purposes only)
-
ammonium chromate (for display purposes only)
![]() Required
equipment:
Required
equipment:
- petri dish
- (optionally) exsiccator and vacuum pump
![]() Safety:
Safety:
- Nitric acid is very corrosive and gives yellow stains on exposed skin. Use gloves when performing the experiments. In case of accidental exposure to the acid, immediately rinse with cold water.
- The chromates and dichromates are toxic and carcinogens. Avoid exposure, especially be careful with possible production of fine dust. The dichromates also may cause skin rashes on certain sensitive persons.
- Red phosphorus is very flammable.
- The decomposition reaction of ammonium dichromate and ammonium trichromate is very nice to see, but be careful not to inhale any of the dust produced in the reactions. This dust contains some leftover hexavalent chromium and hence must be considered toxic.
![]() Disposal:
Disposal:
- Both the solid waste and the solutions must be regarded as highly toxic waste. Any remains of the hexavalent chromium compounds can be neutralized by adding them to a slight excess of an acidified solution of sodium sulfite. This reduces the toxic hexavalent chromium to relatively benign trivalent chromium. Complete reduction has occurred if the solution is purely green blue, without any brown tinge.
- If only small quantities of the salts are used, then the trivalent chromium waste may be flushed down the drain with a large amount of water. If larger quantities (well above 1 gram) are used, then collect the trivalent chromium waste and bring to a proper waste processing facility.
![]()
Preparation of ammonium trichromate
![]() Put 1 ml
of concentrated nitric acid (60% or so) in a test tube and add one big spatula
full of ammonium dichromate. Volume of crystalline solid must be approximately
half the volume of acid. Slightly heat the liquid, until all solid has
dissolved. This results in a dark red solution.
Put 1 ml
of concentrated nitric acid (60% or so) in a test tube and add one big spatula
full of ammonium dichromate. Volume of crystalline solid must be approximately
half the volume of acid. Slightly heat the liquid, until all solid has
dissolved. This results in a dark red solution.
![]() Let the
liquid cool down slowly. If no crystalline solid is formed, then add some more
ammonium dichromate and heat again, until all has dissolved, and then let cool
down again.
Let the
liquid cool down slowly. If no crystalline solid is formed, then add some more
ammonium dichromate and heat again, until all has dissolved, and then let cool
down again.
![]() Wait one
day. After this day, there will be large deep red crystals.
Wait one
day. After this day, there will be large deep red crystals.
![]() Decant
any liquid above the crystals and put the still wet crystals in a petri dish.
Quickly rinse with a tiny amount of very cold concentrated nitric acid and then
carefully dry the crystal mass with a piece of filter paper. Stick the paper in
the crystal mass and let it absorb the liquid from the crystals. The filter
paper becomes dark (black/green) and is partially oxidized. By repeatedly
absorbing liquid in filter paper, the crystals already can be made fairly dry.
Decant
any liquid above the crystals and put the still wet crystals in a petri dish.
Quickly rinse with a tiny amount of very cold concentrated nitric acid and then
carefully dry the crystal mass with a piece of filter paper. Stick the paper in
the crystal mass and let it absorb the liquid from the crystals. The filter
paper becomes dark (black/green) and is partially oxidized. By repeatedly
absorbing liquid in filter paper, the crystals already can be made fairly dry.
![]() Let the
crystals dry in contact with air. Heating above a heat radiator helps drying the
crystals. Even better results are obtained with the help of a vacuum exsiccator,
but without this, also good results should be obtained, provided the humidity of
the air is not too high.
Let the
crystals dry in contact with air. Heating above a heat radiator helps drying the
crystals. Even better results are obtained with the help of a vacuum exsiccator,
but without this, also good results should be obtained, provided the humidity of
the air is not too high.
The final product consists of beautiful red crystals, with a much darker color than the original ammonium dichromate. The solid is not hygroscopic and keeps well, just as ammonium dichromate. The crystals are perfectly dry and do not give off any acid vapor.
It is nice to see how the color of ammonium polychromates darkens with increasing number of chromium atoms in the anion. Below follows a picture with three little vials, at the left there is ammonium chromate (a commercial sample of lab reagent grade), the middle vial contains ammonium dichromate (also a commercial sample, general lab reagent), the right vial contains the crystals, prepared according to the procedure above.

The compound in the right vial probably is ammonium trichromate, but it may also contain some ammonium tetrachromate. With the limited resources, available for testing, the precise composition cannot easily be determined, but one thing is for sure, the compound has a higher chromium content than the dichromate.
![]()
Preparation of potassium trichromate
The process of making potassium trichromate is very similar to the process of making ammonium trichromate. Replace the ammonium dichromate by potassium dichromate and repeat the same procedure.
The crystals, obtained in this way, are smaller than the crystals, obtained from the ammonium dichromate. The solid crystallizes from the liquid much faster, and this causes the resulting crystals to be smaller. The following picture shows potassium chromate at the left, potassium dichromate in the middle, and the result of crystallization from concentrated nitric acid at the right.

All three potassium salts are perfectly dry and keep well. The potassium trichromate, dried in a vacuum exsiccator, does not give off any acid vapors at all.
![]()
All six compounds in a row
It is nice to see, how the color of the different (poly)chromates changes as function of chromium content, but also depending on the cation. Below, a picture is given with all six compounds in a row.

Potassium chromate has the lightest color, it has a really nice bright yellow color. Ammonium chromate has a more golden yellow color. Potassium dichromate and ammonium dichromate have the same color. The potassium trichromate seems to have a slightly lighter color than the ammonium trichromate, but this most likely is due to a somewhat finer division of the solid.
Probably, the golden yellow color of the ammonium chromate is due to an impurity in the form of dichromate ion. Ammonium chromate is not very stable, it slowly looses ammonia and water vapor, when kept in contact with air, leaving ammonium dichromate behind.
![]()
Comparison of oxidizing power of dichromate with trichromate
Solutions of dichromates and trichromates do not differ very much. When a solution of a trichromate is made, then the trichromate decomposes, giving dichromate. Only at very high concentration, combined with highly acidic conditions, the trichromate ion is sufficiently stable to exist in solution. So, the aqueous solution chemistry of the trichromates is not very different from the solution chemistry of the acidified dichromates.
The oxidizing properties of the solids are quite different though. Some of the potassium trichromate was finely ground and mixed with some finely powdered red phosphorus. This mix, when ignited, burns very fast, almost like flash powder.
A similar mix of red phosphorus and finely ground potassium dichromate only has an orange/yellow glow, going through the solid mass, when it is ignited. So, still a very exothermic reaction occurs, but it by far is not as energetic as the trichromate/P mix.
![]()
Decomposition of ammonium di/trichromate
It is well-known that ammonium dichromate decomposes with a red glow (no real fire) and formation of a very large volume of green chromium(III) oxide.

Two videos of this reaction can be downloaded. It is always fun to see this decomposition reaction:
- Decomposition of ammonium dichromate, closeup (appr. 5 MByte)
- Decomposition of ammonium dichromate, overview (appr. 2.7 MByte)
The following two pictures show how much chromium oxide remains after the reaction. Only a small amount initially was in the test tube, a large heap of the green material remains after the experiment.


Ammonium trichromate also decomposes on heating, but the reaction is less violent. This can be explained, because the oxygen/hydrogen balance is optimal for ammonium dichromate, while for ammonium trichromate, there is one excess CrO3 unit, which does not have any 'fuel' in the form of ammonium ion. However, the decomposition is spectacular in another way. Even a small crystal produces a large voluminous 'snake' when it decomposes. The 'snakes' are dark grey. On standing, they become somewhat lighter gray. During decomposition also some orange/brown smoke is formed. This most likely is volatilized CrO3.
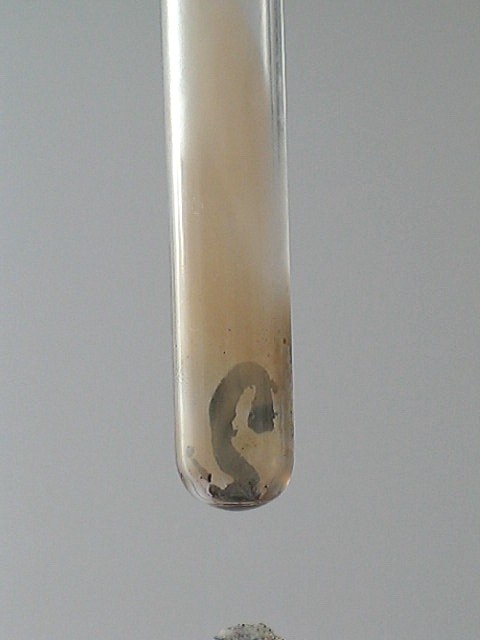
Again, two videos of this reaction can be downloaded:
- Decomposition of ammonium trichromate, one crystal (appr. 2.8 MByte)
- Decomposition of ammonium trichromate, many small crystals (appr. 3.2 MByte)
![]()
Discussion of results
Hexavalent chromium salts are derived from the oxide CrO3, and the corresponding acid H2CrO4. This acid does not exist in the pure state, in fact, it only is a hypothetical acid.
![]() At sufficiently high pH, hexavalent chromium only
exists as chromate, CrO42-. In solution, this ion is in
equilibrium as follows:
At sufficiently high pH, hexavalent chromium only
exists as chromate, CrO42-. In solution, this ion is in
equilibrium as follows:
CrO42- + H2O ↔ HCrO4– + OH–
The ion HCrO4– in turn is in equilibrium as follows:
2HCrO4– ↔ Cr2O72- + H2O
When the pH is decreased, then the equilibrium more and more shifts towards the direction of dichromate ion, Cr2O72-.
The equilibria, shown above, are very labile. A change of pH immediately is followed by a change of concentration of chromate or dichromate. This kind of equilibria can nicely be demonstrated. When a yellow solution of a chromate is acidified, then it becomes orange, due to the conversion to dichromate. If the solution is made alkaline again, then it becomes yellow again, due to the conversion to chromate.
![]() When the pH is made very low, and at the same time,
the concentration of dichromate is made very high, then further condensation
occurs.
When the pH is made very low, and at the same time,
the concentration of dichromate is made very high, then further condensation
occurs.
At really low pH, the dichromate is in equilibrium as follows:
Cr2O72- + H+ ↔ HCr2O7–
Two of such ions can condense into a tetrachromate ion:
2HCr2O7– ↔ Cr4O132- + H2O
The tetrachromate ion is very unstable and easily looses a molecule of CrO3.
Cr4O132- → Cr3O102- + CrO3
The CrO3 immediately forms dichromate through H2CrO4, or it adds to an existing dichromate ion.
The net result of these equilibria is that the solution mainly contains trichromate, Cr3O102-. The color of such solutions is deep red, much darker than the color of concentrated solutions of dichromates.
![]() From the highly concentrated solutions, where the trichromate ion is the
dominant chromium (VI) species, the trichromate crystallizes.
From the highly concentrated solutions, where the trichromate ion is the
dominant chromium (VI) species, the trichromate crystallizes.
It might even be so, that some of the tetrachromate crystallizes from the solutions, but that is not easily checked without advanced equipment. The red solids, shown above, most likely are trichromates, but they may also contain some tetrachromate.
From the highly concentrated solutions, where the trichromate ion is the dominant chromium (VI) species, the trichromate crystallizes.
![]() Ammonium dichromate has optimal oxygen/hydrogen balance for decomposition. The
decomposition reaction is as follows:
Ammonium dichromate has optimal oxygen/hydrogen balance for decomposition. The
decomposition reaction is as follows:
(NH4)2Cr2O7 → N2 + 4H2O + Cr2O3 (+ heat)
For decomposition of the trichromate, there is a suboptimal condition. The compound has excess units of CrO3. Most likely, the main reaction is as follows:
(NH4)2Cr3O10 → N2 + 4H2O + Cr2O3 + CrO3 (+ heat),
where the CrO3 for a small part volatilizes, giving rise to the brown/orange smoke, but most part is expected to decompose at the high temperatures of decomposition, giving rise to oxygen, and a mix of oxides, like Cr2O3, CrO2 and other intermediates, containing chromium(III), (IV) and (VI). This most likely explains, why the 'snakes' are black/dark grey and not green. The 'snakes' are insoluble in water and in concentrated HCl. They are extremely delicate. Even careful touching makes them disintegrate. This shows that they are mostly gas and in fact the snakes are a kind of solid 'foam' with very low structural strength.
Rinsing of the snakes also results in disintegration, most of the material being insoluble, but some orange solution can be obtained. This shows that they still contain appreciable amounts of hexavalent chromium.
![]()
Structure of (poly)chromates
The polychromates can be thought of as chains of CrO4-units, attached to each other by means of a shared oxygen atom. Below follow pictures of the structures of the chromate ion, dichromate ion and trichromate ion. Red spheres denote oxygen, grey spheres denote chromium.
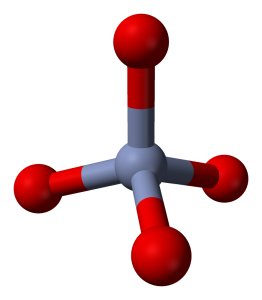
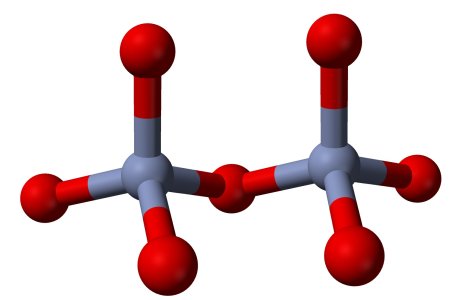
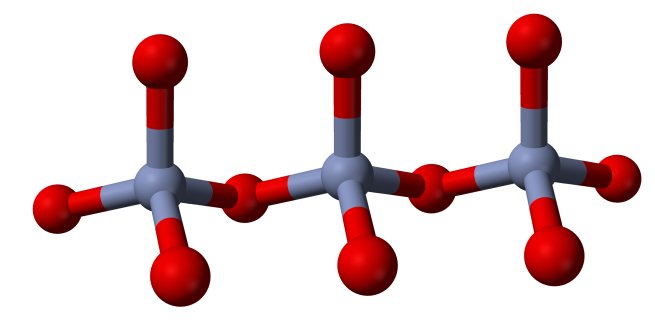
The information about these polychromate ions is from the book "Chemistry of the Elements", third edition, written by Greenwood and Earnshaw.