


Lead(II) iodide -- nice experiments
The compound lead(II) iodide has a few remarkable properties and this compound allows some neat experiments to be done.
The most remarkable is the color of the compound, as function of temperature. Lead(II) iodide can show every color between bright yellow and brick red, depending on temperature. Color changes are reversible. On cooling down after heating, the compound get its normal yellow color again.
When lead(II) iodide is heated more strongly, then it melts and slowly decomposes. Strangely, hardly any free lead seems to form, but some brown colored substance, which may be lead(II) iodide, mixed with lead, or even with lead, dissolved in it. The brown substance, formed in the decomposition reaction, may also be due to dissolving of iodine in lead(II) iodide, while it is still liquid.
 This
is a nice experiment, but one has to be very careful with the lead(II)
compounds. Unfortunately, these are very poisonous. Lead(II) ions are a
cumulative poison. Multiple small doses, spread out over a long period of time
add up to form dangerous doses. So, when performing this experiment, be
particularly careful not to be exposed to the lead(II) compounds.
This
is a nice experiment, but one has to be very careful with the lead(II)
compounds. Unfortunately, these are very poisonous. Lead(II) ions are a
cumulative poison. Multiple small doses, spread out over a long period of time
add up to form dangerous doses. So, when performing this experiment, be
particularly careful not to be exposed to the lead(II) compounds.
![]()
![]() Required
chemicals for first experiment:
Required
chemicals for first experiment:
-
lead(II) iodide (can be prepared easily, see here)
-
concentrated nitric acid, 50% or better
![]() Required
equipment:
Required
equipment:
-
test tube
-
some means to safely heat the test tube
![]() Safety:
Safety:
-
 Lead(II) salts are very toxic.
Lead(II) salts are very toxic. -
 Concentrated nitric acid is very
corrosive. If this comes in contact with the skin, then rinse away
immediately with a lot of water. Hot concentrated nitric acid is extremely
corrosive. Under no circumstance, this may come in contact with the skin or
any other body part. Wear goggles and gloves!
Concentrated nitric acid is very
corrosive. If this comes in contact with the skin, then rinse away
immediately with a lot of water. Hot concentrated nitric acid is extremely
corrosive. Under no circumstance, this may come in contact with the skin or
any other body part. Wear goggles and gloves! -
A small amount of iodine vapor and nitrogen dioxide may be released in the air. It is best to do this experiment in a well-ventilated room.
![]() Disposal:
Disposal:
-
The waste must be regarded as toxic chemical waste. Bring it to a proper waste processing facility, do not rinse down the drain.
![]()
Careful heating of bright yellow PbI2
Put some solid lead iodide in a test tube and carefully heat the test tube over a flame, while shaking all the time, in order to avoid strong heating of a single spot.
While the solid is heated, the color shifts from bright yellow to brick red, through bright orange and bright red. The following four pictures show the initial lead(II) iodide, and the heated substance.




When the solid cools down, then it becomes yellow again. If the heating is too strong, then the color change is not reversible anymore. Strong heating causes the substance to melt, and then decompose. That is demonstrated in the next experiment.
![]()
Melting and decomposition of PbI2
When the heating is continued, then the lead(II) iodide melts and a dark liquid is formed, which has a very shiny surface, almost like metal. The picture shows the dark liquid and the metal-like surface of this liquid. Above the liquid, a faint purple color of iodine vapor can be observed. That is due to slight decomposition. As soon as lead(II) iodide melts, the first weak signs of decomposition can be observed.

On much stronger heating in the flame of a propane torch, the decomposition is stronger. In that case, the liquid becomes even darker, almost black and a deep purple vapor of iodine is emitted from the liquid. In the higher and cooler parts of the test tube, the iodine forms crystals at the glass.

The liquid can be poured down along the glass of the test tube, and while doing so, it solidifies again. The left picture below shows the partially cooled down test tube, after attempting to pour some of the dark liquid out of the test tube. The liquid solidified while pouring, and a little spoon-shaped object was formed, along the glass, which has a metal-like luster. The liquid at the bottom of the test tube has solidified also and has become much lighter again. When the test tube further cools down, then the spoon-like structure does not stick to the glass anymore. The end of it settles at the middle of the test tube, in free air. The lead iodide also becomes more and more yellow, while cooling down. The right picture shows that the lead iodide has become yellow again.


The pictures below show the spoon-shaped structure, which is not sticking to the glass anymore and could easily be removed after cooling down.


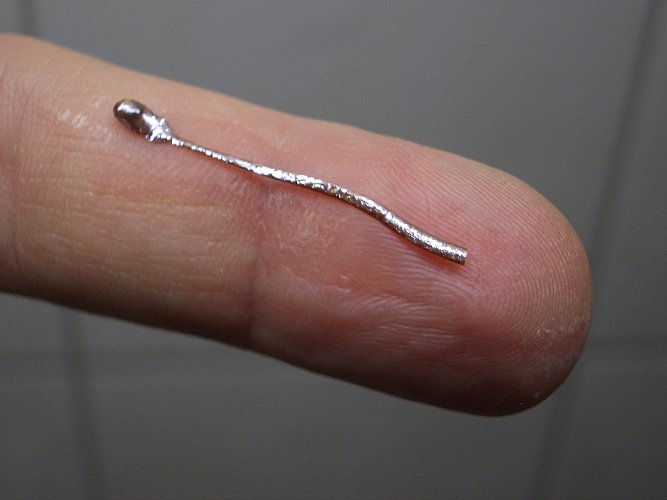
The little spoon-like structure mostly is lead(II) iodide, probably with small amounts of lead mixed with it. This little structure is very brittle, and the head broke from the tail. This object was put in some boiling water and after that, it was put on a glass petri dish with some water, such that it sticks to the glass and can be photographed. The head is put on the glass, with the bottom and top side exchanged, such that the difference between the bottom and top sides is best demonstrated.
Below follow two pictures of the structure. The yellow and smooth side was formed on the glass, the other red/brown side, covered with metal-like specks was formed in contact with the air and iodine vapor. When viewed in this way, the shape of this structure resembles the shape of a sperm cell.


Most likely, the metallic looking spots on the side, which was in contact with air, are pieces of lead metal. The yellow side of the structure is lead(II) iodide.
![]()
Oxidizing with nitric acid
The 'sperm cell' was put back in the test tube, with the remaining lead(II) iodide and some 50% nitric acid was added. When this is added, then bubbles of gas are produced at the surface of the 'sperm cell'. This most likely is due to dissolving of lead on its surface. The picture below shows the formation of the bubbles and it shows that the air in the test tube becomes brown, due to formation of NO2.

When the test tube with lead(II) iodide and nitric acid is heated, then a beautiful wine-red gas mixture is produced and all of the solid dissolves. This gas mixture really is beautiful. This beautiful red color is caused by a mix of iodine vapor and nitrogen dioxide. The purple and brown mix to form a deep red color. At the higher and cooler parts of the test tube, iodine crystallizes on the glass.


On cooling down, a white crystalline mass settles at the bottom. This crystalline mass is lead nitrate. Click on the pictures below for a more detailed picture, especially the glittering crystals of lead nitrate, which settle from the liquid are very nice to see.
![]()
Discussion of results
![]() The color of lead(II) iodide depends on temperature. It is a covalent compound
and that also explains why its properties are not a simple mix of the properties
of iodide ions and lead(II) ions. Lead(II) iodide as covalent compound has
totally different properties than the ions, from which it is formed.
The color of lead(II) iodide depends on temperature. It is a covalent compound
and that also explains why its properties are not a simple mix of the properties
of iodide ions and lead(II) ions. Lead(II) iodide as covalent compound has
totally different properties than the ions, from which it is formed.
![]() Lead(II) iodide decomposes on strong heating:
Lead(II) iodide decomposes on strong heating:
PbI2 → Pb + I2
When lead(II) iodide is molten and cooled down again, then the solid becomes yellow again, but with brown/red remains in it. That brown/red material probably is due to dissolved iodine.
![]() The remarkable red gas mix is a real mix. Its color is the resultant of
mixing a brown gas with a deep purple gas.
The remarkable red gas mix is a real mix. Its color is the resultant of
mixing a brown gas with a deep purple gas.
Lead(II) iodide is oxidized by concentrated nitric acid as follows:
PbI2 + 4HNO3 → Pb2+ + 2NO3– + 2NO2 + I2 + 2H2O
The lead nitrate is only sparingly soluble in the concentrated acid and it settles in the form of many small glittering crystals of Pb(NO3)2.


