


Nitration of white cotton wadding
A very nice and spectacular experiment is the conversion of plain white cotton wadding to guncotton. The guncotton, made in this experiment, burns with a very fast and bright flame and not a single residue remains when it burns, and no smoke is produced.
 This
experiment can be safely conducted, but the mix of concentrated nitric acid and
concentrated sulphuric acid, used to prepare the nitrated cotton wadding is
very corrosive. It is absolutely mandatory to wear acid-resistant gloves
during the part of the experiment in which the acids are mixed and in which the
nitrated cotton wadding is taken out of the acid mix. On bare skin, the
nitrating acid mix gives severe wounds within seconds!
This
experiment can be safely conducted, but the mix of concentrated nitric acid and
concentrated sulphuric acid, used to prepare the nitrated cotton wadding is
very corrosive. It is absolutely mandatory to wear acid-resistant gloves
during the part of the experiment in which the acids are mixed and in which the
nitrated cotton wadding is taken out of the acid mix. On bare skin, the
nitrating acid mix gives severe wounds within seconds!
![]()
![]() Required
chemicals:
Required
chemicals:
-
concentrated sulphuric acid (95% or better)
-
concentrated nitric acid (60% to 70%)
-
white cotton wadding
-
sodium bicarbonate
![]() Required
equipment:
Required
equipment:
-
test tubes
-
glass rod
-
test tube
![]() Safety:
Safety:
- Concentrated nitric acid and sulphuric acid are very corrosive. The mix, prepared in this experiment is even more corrosive.
- There is a (small) chance of runaway in this experiment. Keep a bucket of water nearby, just in case the mix heats up and is going into a runaway condition.
![]() Disposal:
Disposal:
- The used nitrating mix can be diluted with a lot of water and then be flushed down the drain.
![]()
Preparation of the nitrating acid mix
![]() Take
approximately 3 ml of concentrated sulphuric acid and put that in a clean and
dry test tube.
Take
approximately 3 ml of concentrated sulphuric acid and put that in a clean and
dry test tube.
![]() Take
approximately 1.5 ml of concentrated nitric acid and put that in another test
tube.
Take
approximately 1.5 ml of concentrated nitric acid and put that in another test
tube.

![]() Carefully add the sulphuric acid to the nitric acid. Do not add all acid at once,
but add it in 4 steps, shaking well between the steps. Each time, let the mix
cool down a little. Quite some heat is produced, when the nitric acid and
sulphuric acid are mixed. The amount of heat is not as high, when sulphuric acid
and water are mixed, but nevertheless, be careful. After mixing of all acid, let
the mix cool down to room temperature.
Carefully add the sulphuric acid to the nitric acid. Do not add all acid at once,
but add it in 4 steps, shaking well between the steps. Each time, let the mix
cool down a little. Quite some heat is produced, when the nitric acid and
sulphuric acid are mixed. The amount of heat is not as high, when sulphuric acid
and water are mixed, but nevertheless, be careful. After mixing of all acid, let
the mix cool down to room temperature.
After the mixing of the acids, a colorless fairly mobile liquid is obtained, which fumes a little in contact with air. Be careful not to breathe the fume.
![]()
Nitration of a piece of wadding
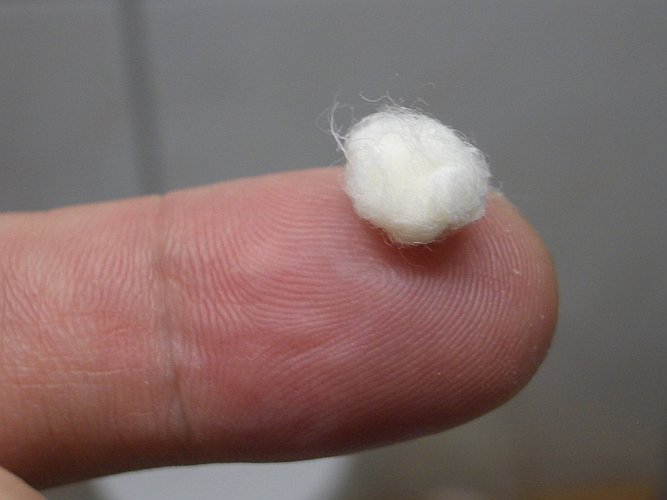
![]() Take a small amount of white cotton wadding. The piece of
wadding should not be too large and it should not be impressed too much. The
following picture shows a piece of wadding, which perfectly matches for this
experiment.
Take a small amount of white cotton wadding. The piece of
wadding should not be too large and it should not be impressed too much. The
following picture shows a piece of wadding, which perfectly matches for this
experiment.

![]() Put the
piece of wadding at the top of the test tube and with a glass rod press it into
the acid mix. It must be covered by the acid mix completely. Quickly after the
wadding comes in contact with the acid, it becomes a little bit off-white, it
obtains a very light yellow tint.
Put the
piece of wadding at the top of the test tube and with a glass rod press it into
the acid mix. It must be covered by the acid mix completely. Quickly after the
wadding comes in contact with the acid, it becomes a little bit off-white, it
obtains a very light yellow tint.

Keep the piece of wadding in the acid for 20 to 25 minutes and every few minutes shake well, such that the acid is swirled around thoroughly, but be very careful not to spill any acid over the rim of the test tube.
![]() If during this phase of the experiment there is noticeable darkening (bright
yellow, orange or brown coloration) of the wadding or liquid, then
immediately dump the test tube in a bucket full of water. Darkening is an
indication of onset to runaway. A runaway of this nitration reaction is very
rare, but it is possible (there are reports of such a runaway reaction), so be
prepared for such a condition.
If during this phase of the experiment there is noticeable darkening (bright
yellow, orange or brown coloration) of the wadding or liquid, then
immediately dump the test tube in a bucket full of water. Darkening is an
indication of onset to runaway. A runaway of this nitration reaction is very
rare, but it is possible (there are reports of such a runaway reaction), so be
prepared for such a condition.
![]() After 20
to 25 minutes, decant the nitration mix from the piece of wadding. With some
patience, most of the acid mix can be recovered and it could be used for a
second, somewhat smaller batch. If there is no desire to make a second batch,
then decant the acid mix in 100 ml of water, stir a little bit and flush down
the drain.
After 20
to 25 minutes, decant the nitration mix from the piece of wadding. With some
patience, most of the acid mix can be recovered and it could be used for a
second, somewhat smaller batch. If there is no desire to make a second batch,
then decant the acid mix in 100 ml of water, stir a little bit and flush down
the drain.
![]() The
nitrated cotton wadding, still soaked with acid mix must be dumped in a beaker
or erlenmeyer with approximately 100 ml of water. Swirl the liquid around, such
that most of the acid is rinsed away from the piece of wadding.
The
nitrated cotton wadding, still soaked with acid mix must be dumped in a beaker
or erlenmeyer with approximately 100 ml of water. Swirl the liquid around, such
that most of the acid is rinsed away from the piece of wadding.

![]() Decant the acidic water and add a fresh amount of water.
Again swirl the mix around. Repeat this a few times. After this, take out the
piece of wadding, and tear it apart, such that its interior part also is exposed
well to water. Then put it in the erlenmeyer again, with fresh water.
Decant the acidic water and add a fresh amount of water.
Again swirl the mix around. Repeat this a few times. After this, take out the
piece of wadding, and tear it apart, such that its interior part also is exposed
well to water. Then put it in the erlenmeyer again, with fresh water.

![]() Add a pinch of sodium bicarbonate, and dissolve this, while
the piece of wadding still is in the water and swirl the erlenmeyer around for a
while. No bubbles of carbon dioxide may be observed, otherwise rinse a few times
again. After the rinse with sodium bicarbonate, rinse again with water and then
take out the piece of wadding.
Add a pinch of sodium bicarbonate, and dissolve this, while
the piece of wadding still is in the water and swirl the erlenmeyer around for a
while. No bubbles of carbon dioxide may be observed, otherwise rinse a few times
again. After the rinse with sodium bicarbonate, rinse again with water and then
take out the piece of wadding.
![]() Press the piece of wadding into a small ball. In this way,
most of the water is removed already. You can also feel, that the piece of
wadding is not as soft anymore, as it was before the experiment. The fibers are
a little bit rougher and a little bit more brittle. The picture below shows the
size of the small ball of wadding.
Press the piece of wadding into a small ball. In this way,
most of the water is removed already. You can also feel, that the piece of
wadding is not as soft anymore, as it was before the experiment. The fibers are
a little bit rougher and a little bit more brittle. The picture below shows the
size of the small ball of wadding.
![]() Tear the ball of wadding apart and let it dry in a clean and
dry place. After a few hours of drying at room temperature, it is ready for use.
The pictures below show the size of the piece of wadding, after it is torn
apart. Do the drying in a place, where accidental ignition of the piece of
wadding does no harm. Also, if you intend to store some of the nitrated pieces
of wadding, store them in a place, where accidental ignition does not cause fire
(e.g. in a metal tin, loosely capped).
Tear the ball of wadding apart and let it dry in a clean and
dry place. After a few hours of drying at room temperature, it is ready for use.
The pictures below show the size of the piece of wadding, after it is torn
apart. Do the drying in a place, where accidental ignition of the piece of
wadding does no harm. Also, if you intend to store some of the nitrated pieces
of wadding, store them in a place, where accidental ignition does not cause fire
(e.g. in a metal tin, loosely capped).


![]()
Burning of the wadding
The piece of wadding can be lit in many ways. A glowing cigarette is sufficient to make it inflame. Be careful with lighting a large piece. The flame from a small piece of wadding is remarkably large. A small movie is made of the burning of a small piece of wadding (approximately ⅓ part of the quantity shown above). Below follow a few frames of this movie:












This sequence shows the big flame, and it also shows that no residue remains at all. No smoke is produced, and no ashes remain behind. This is a remarkable property of this nitrated cotton wadding. The sequence, shown above, only covers an interval of time of 0.4 seconds. This also shows that the burning of the nitrated cotton wadding is very fast.
There also is a small AVI movie. Click here for the animation. File size is approximately 800 kByte, download speed is limited to 50 kByte per second. For some systems, the animation does not play by simply clicking it. If that is the case on your system, then first save the file to your local harddisk and open it from that place.
![]()
Discussion of the results
White cotton wadding is a form of very pure cellulose. Cellulose is a polymeric chain, derived from beta-glucose. It has empirical formula (C6H10O5)n. Its structure is as follows:
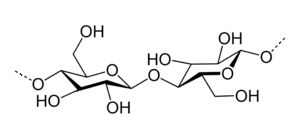
This is a chain of glucose-units, connected by means of oxygen atoms.
The nitric acid in the nitrating mix forms an ester on each -OH group of the cellulose polymer. This esterification reaction is an equilibrium reaction:
R-OH + HO-NO2 ↔ R-O-NO2 + H2O
Here R is the glucose-core, to which the -OH group is connected. This reaction can take place on all -OH groups in the cellulose polymer. The concentrated sulphuric acid is needed for driving this reaction to the right, by taking away the water molecules, formed in this reaction. The resulting chemical has the following structure:
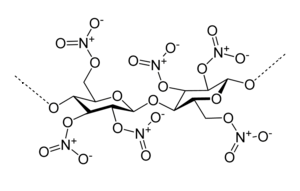
Each -OH group is replaced by an -O-NO2 group.
Normal cellulose is flammable, but it only burns slowly and leaves behind carbonated remnants. Only at very high temperatures, e.g. in the flame of a propane torch, one can get rid of all carbon remains from burning cellulose. However, when it is fully nitrated, then the oxidizer already is present in the polymer itself.