


A remarkable nickel(I) complex
In aqueous chemistry, nickel almost exclusively is in the +2 oxidation state. Most common is the green aqua complex of nickel(II), but there are many other nickel(II) complexes, most of them having a green or a bluish color, although there also are some yellow and red complexes (e.g. the dimethylglyoxime complex).
In this experiment, a nickel(I) complex is prepared. This oxidation state is very uncommon for nickel.


 The
chemicals used in this experiment are very toxic. Nickel salts are carcinogens
and the cyanide ligand, used in this experiment is extremely toxic. Doses of 200
mg or so can be fatal. The solutions, used in this experiment are also strongly
alkaline and this strong alkalinity makes the toxic properties of the liquid
even more dangerous (easy absorption of cyanide through damaged skin). Avoid
skin-contact with the liquid at any cost.
The
chemicals used in this experiment are very toxic. Nickel salts are carcinogens
and the cyanide ligand, used in this experiment is extremely toxic. Doses of 200
mg or so can be fatal. The solutions, used in this experiment are also strongly
alkaline and this strong alkalinity makes the toxic properties of the liquid
even more dangerous (easy absorption of cyanide through damaged skin). Avoid
skin-contact with the liquid at any cost.
![]()
![]() Required
chemicals:
Required
chemicals:
-
nickel sulfate (do not use the ammonium nickel salt, the ammonium interferes)
-
potassium cyanide (sodium cyanide is suitable as well)
-
sodium hydroxide (potassium hydroxide is suitable as well)
-
sulphuric acid
-
low boiling ligroin (or any other inert organic solvent like pentane, hexane)
-
hydrogen peroxide
-
bleach (for destruction of cyanide and safe disposal of waste)
![]() Required
equipment:
Required
equipment:
- test tubes
- little alcohol burner or other small burner for heating test tubes
- pasteur pipette with long needle-like tip
![]() Safety:
Safety:
- Nickel salts are known carcinogens, avoid exposure.
 Potassium cyanide is extremely toxic and doses of
200 mg can be fatal.
Potassium cyanide is extremely toxic and doses of
200 mg can be fatal.- Sodium hydroxide is very corrosive and especially, when combined with cyanide this can be really dangerous, due to risk of absorption of cyanide through damaged skin.
- At the end of the experiment, a cyanide-containing solution is acidified, which leads to formation of hydrogen cyanide. The amount of gas, released in the air is very low (if any at all), but just to be sure, perform the experiment in a very well ventilated room, or even better in a fume hood.
![]() Disposal:
Disposal:
- The cyanide content can be destroyed by adding bleach to the solutions and keeping pH high. Wait a few minutes after adding the bleach, then all cyanide is destroyed and all nickel compounds have turned black. After this step, the solution should be treated as heavy metal waste, the waste should not be flushed down the drain, but be brought to a proper waste processing facility.
![]()
Preparation of a cyano nickel(II) complex
The fairly well-known tetracyanonickelate(II) complex serves as a starting point for this experiment. This complex easily can be made in aqueous solution from nickel sulfate and potassium cyanide.
![]() Prepare
a moderately concentrated solution of nickel(II) sulfate. This solution is clear
and has a nice green color.
Prepare
a moderately concentrated solution of nickel(II) sulfate. This solution is clear
and has a nice green color.

![]() To the
green solution carefully drip a fairly concentrated solution of potassium
cyanide. After each drop, the solution should be swirled. First, a dirty green
precipitate is formed, but this redissolves when excess cyanide ion is present.
Keep on adding potassium cyanide, such that the precipitate just redissolves.
Avoid using too much cyanide. When just enough cyanide is added then a yellow
solution is obtained. This solution may be slightly turbid. If this is the case,
then one can allow the solid matter to settle at the bottom.
To the
green solution carefully drip a fairly concentrated solution of potassium
cyanide. After each drop, the solution should be swirled. First, a dirty green
precipitate is formed, but this redissolves when excess cyanide ion is present.
Keep on adding potassium cyanide, such that the precipitate just redissolves.
Avoid using too much cyanide. When just enough cyanide is added then a yellow
solution is obtained. This solution may be slightly turbid. If this is the case,
then one can allow the solid matter to settle at the bottom.

If too much cyanide is added, then another complex is formed, which has a somewhat brown/orange color and this makes observation of the nickel(I) complex harder. If accidently too much cyanide is added, then add some nickel(II) sulfate again, until the solution just remains turbid. Then the turbidity can be filtered off, or one has to wait till the solid matter settles at the bottom and then the clear liquid can be decanted.
![]()
Preparation of the nickel(I) complex
![]() Once the
tetracyanonickelate(II) complex is formed and a clear solution is obtained, the
solution must be boiled briefly. This is necessary to drive off the dissolved
oxygen from the air. Let the liquid cool down. In the meantime, the next step
can be performed.
Once the
tetracyanonickelate(II) complex is formed and a clear solution is obtained, the
solution must be boiled briefly. This is necessary to drive off the dissolved
oxygen from the air. Let the liquid cool down. In the meantime, the next step
can be performed.
![]() In a
separate test tube heat some low boiling ligroin (boiling range 40...60 °C) or
other volatile alkane-based solvent (e.g. pentane, hexane). Do not use an open
flame, but keep the test tube with this solvent in a beaker filled with boiling
hot water. This treatment drives off dissolved oxygen from the solvent.
In a
separate test tube heat some low boiling ligroin (boiling range 40...60 °C) or
other volatile alkane-based solvent (e.g. pentane, hexane). Do not use an open
flame, but keep the test tube with this solvent in a beaker filled with boiling
hot water. This treatment drives off dissolved oxygen from the solvent.
![]() Pour the
solvent on the cooled down yellow solution such that a thick layer of solvent is
above the aqueous layer. This prevents oxygen from the air to reach the aqueous
layer.
Pour the
solvent on the cooled down yellow solution such that a thick layer of solvent is
above the aqueous layer. This prevents oxygen from the air to reach the aqueous
layer.
After this step you have a test tube with a yellow liquid as bottom layer and the colorless ligroin layer on top of it. Now the actual preparation of the complex can be started.
![]() Add a
small spatula full of coarse zinc powder or small zinc granules to the test tube
with the two layers. Try to add the zinc such that it does not stick to the
glass wall above the ligroin layer. Immediately after this, add a somewhat
bigger spatula full of sodium hydroxide. Again avoid sticking of the solid to
the glass.
Add a
small spatula full of coarse zinc powder or small zinc granules to the test tube
with the two layers. Try to add the zinc such that it does not stick to the
glass wall above the ligroin layer. Immediately after this, add a somewhat
bigger spatula full of sodium hydroxide. Again avoid sticking of the solid to
the glass.
Both solids quickly fall through the ligroin layer and most will fall through the aqueous layer as well. If some particles remain stuck at the water/ligroin interface, then carefully tap the test tube, such that all solid material goes to the bottom.
The sodium hydroxide quickly dissolves and a local hot spot is formed at the bottom. Swirl the test tube somewhat, but do not shake. The ligroin layer always must remain on top of the aqueous layer. Every few seconds you need to swirl again, so that fresh solution with the yellow nickel(II) complex can reach the zinc particles. The zinc dissolves in the strongly alkaline liquid, giving bubbles of hydrogen gas. But at the same time, the yellow nickel(II) complex is reduced and a deep red nickel(I) complex is formed. After a few tens of seconds and a few times of swirling, the situation is as follows:

At the bottom there is the zinc, mixed with remains of solid sodium hydroxide. Small bubbles of gas are formed, which somewhat collect near the water/ligroin interface, but many tiny bubbles also escape through the ligroin layer. Initially, the liquid seems to turn orange, but when most of the zinc has dissolved, then the true intense color of the complex can be observed. It is deep red.
A little more ligroin was added just to assure that it takes a long time for oxygen from the air to reach the aqueous layer. After 15 minutes or so, the liquid is deep red and small bubbles of gas have collected near the ligroin/water interface.

![]() The
liquid was diluted somewhat with pre-boiled water to make the color of the
liquid somewhat less intense, such that color nuances could be observed more
easily. After a day, the diluted liquid also has a much sharper interface with
the ligroin layer. All little gas bubbles have gone and a two layer system is
obtained with a deep red bottom layer and a colorless upper layer.
The
liquid was diluted somewhat with pre-boiled water to make the color of the
liquid somewhat less intense, such that color nuances could be observed more
easily. After a day, the diluted liquid also has a much sharper interface with
the ligroin layer. All little gas bubbles have gone and a two layer system is
obtained with a deep red bottom layer and a colorless upper layer.

![]()
Some properties of the nickel(I) complex
The nickel(I) complex was allowed to stand for several days, with the ligroin layer above the aqueous layer and the test tube was loosely stoppered with a cork. The complex seems to be fairly stable. Even after 5 days, no noticeable changes could be observed. The complex still is deep red and the liquid still is clear. No precipitate had settled at the bottom.
![]() Some of the liquid was taken from below the ligroin layer
with the help of a pasteur pipette and transferred to another clean test tube,
which was shaken every few seconds, such that there is contact with fresh air.
When this is done, then the liquid changes color very quickly. It really is
amazing to see the change of color occurring so fast. In approximately 1 minute,
the color has gone from deep red to pale yellow. The top-left picture was made,
immediately after transferring the liquid, the top-right picture was made 20
seconds later, with two shakes in between. The bottom left-picture was made 40
seconds later and the final picture was made approximately 1 minute later.
Some of the liquid was taken from below the ligroin layer
with the help of a pasteur pipette and transferred to another clean test tube,
which was shaken every few seconds, such that there is contact with fresh air.
When this is done, then the liquid changes color very quickly. It really is
amazing to see the change of color occurring so fast. In approximately 1 minute,
the color has gone from deep red to pale yellow. The top-left picture was made,
immediately after transferring the liquid, the top-right picture was made 20
seconds later, with two shakes in between. The bottom left-picture was made 40
seconds later and the final picture was made approximately 1 minute later.




So, in the presence of oxygen from the air, the complex is oxidized very quickly, while it remained stable for several days under the layer of ligroin.
![]() Another
interesting experiment is the addition of acid to the liquid. An excess amount
of dilute sulphuric acid (approximately 2 M) was added to part of the deep red
liquid and this results in immediate formation of a yellow/ochre precipitate.
Another
interesting experiment is the addition of acid to the liquid. An excess amount
of dilute sulphuric acid (approximately 2 M) was added to part of the deep red
liquid and this results in immediate formation of a yellow/ochre precipitate.

The exact nature of this precipitate is unsure. Most likely it is a precipitate of zinc ions with the nickel(I) complex. In the strongly alkaline conditions, the zinc remains in solution as zincate, but in acidic solution this is converted to zinc(II) cation, which gives a precipitate with the nickel(I) complex.
![]() When a small amount of the yellow precipitate is poured in
another test tube and diluted with water (not pre-boiled), then it does not
change. The yellow color remains, even when it is shaken:
When a small amount of the yellow precipitate is poured in
another test tube and diluted with water (not pre-boiled), then it does not
change. The yellow color remains, even when it is shaken:
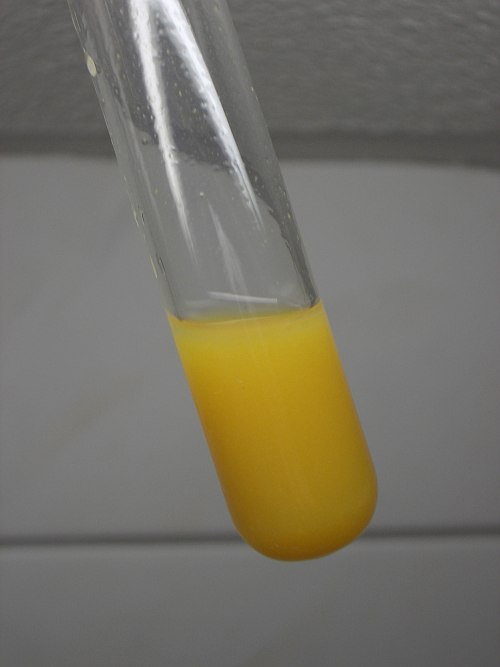
![]() All of
the yellow precipitate (contents of both test tubes with yellow precipitate) was
dumped in an erlenmeyer, filled with a very dilute solution of hydrogen peroxide
which also was slightly acidified. When this is done, then the yellow
precipitate at once turns off-white and a milk-like liquid is obtained with a
faint greenish hue. When this liquid is allowed to stand for a few hours, then
the precipitate settles at the bottom.
All of
the yellow precipitate (contents of both test tubes with yellow precipitate) was
dumped in an erlenmeyer, filled with a very dilute solution of hydrogen peroxide
which also was slightly acidified. When this is done, then the yellow
precipitate at once turns off-white and a milk-like liquid is obtained with a
faint greenish hue. When this liquid is allowed to stand for a few hours, then
the precipitate settles at the bottom.

So, hydrogen peroxide at once oxidizes the yellow precipitate and a new precipitate is formed. This new precipitate is nickel(II) cyanide. Nickel(II) cyanide is formed when the tetracyanonickelate(II) is acidified.
![]()
Discussion of results
![]() When nickel(II) ions are brought in contact with cyanide ion, then a precipitate
is formed:
When nickel(II) ions are brought in contact with cyanide ion, then a precipitate
is formed:
Ni2+(aq) + 2CN–(aq) → Ni(CN)2(s)
![]() When more cyanide ion is added, then this precipitate redissolves again, giving
the golden yellow tetracyanonickelate(II) complex:
When more cyanide ion is added, then this precipitate redissolves again, giving
the golden yellow tetracyanonickelate(II) complex:
Ni(CN)2(s) + 2CN–(aq) → Ni(CN)42–(aq)
![]() The tetracyanonickelate(II) complex is stable at high pH, but at low pH, it
decomposes and a light green precipitate of Ni(CN)2 is formed again.
The last two cyanide ligands are not easily removed from the nickel ion, not
even by fairly strongly acidic solutions. This is why a precipitate of nickel
cyanide is formed and not a clear solution with aqueous nickel(II) and HCN.
The tetracyanonickelate(II) complex is stable at high pH, but at low pH, it
decomposes and a light green precipitate of Ni(CN)2 is formed again.
The last two cyanide ligands are not easily removed from the nickel ion, not
even by fairly strongly acidic solutions. This is why a precipitate of nickel
cyanide is formed and not a clear solution with aqueous nickel(II) and HCN.
Ni(CN)42–(aq) + 2H+(aq) → Ni(CN)2(s) + 2HCN(aq)
![]() The nickel(II) complex can be reduced by nascent
hydrogen in alkaline conditions. This nascent hydrogen can be prepared
conveniently by means of zinc metal. The nascent hydrogen in turn reduces the
complex. As a side reaction, molecular hydrogen is formed as well:
The nickel(II) complex can be reduced by nascent
hydrogen in alkaline conditions. This nascent hydrogen can be prepared
conveniently by means of zinc metal. The nascent hydrogen in turn reduces the
complex. As a side reaction, molecular hydrogen is formed as well:
Zn + 2OH– → ZnO22– + 2[H]
[H] + Ni(CN)42– + OH– → Ni(CN)32– + CN– + H2O
2[H] → H2
Here [H] stands for nascent hydrogen. This is an intermediate species, which immediately either reacts with something in solution, or forms molecular hydrogen gas.
The complex Ni(CN)32– has a deep red color.
![]() When this liquid is acidified, then a yellow/ochre precipitate is formed. Most
likely this is the zinc-salt of the complex ion. Zinc(II) is present as zincate
in alkaline solution, but in acidic solution this is present as Zn2+.
This precipitates with the nickel(I) complex as Zn[Ni(CN)3]. This
precipitate is not as air-sensitive as the aqueous alkaline solution. Even when
it is shaken in contact with air, the precipitate does not disappear, nor change
color.
When this liquid is acidified, then a yellow/ochre precipitate is formed. Most
likely this is the zinc-salt of the complex ion. Zinc(II) is present as zincate
in alkaline solution, but in acidic solution this is present as Zn2+.
This precipitates with the nickel(I) complex as Zn[Ni(CN)3]. This
precipitate is not as air-sensitive as the aqueous alkaline solution. Even when
it is shaken in contact with air, the precipitate does not disappear, nor change
color.
![]() On addition of hydrogen peroxide, the precipitate at once is oxidized. In the
fairly strongly acidic conditions of the experiment, the nickel at once forms a
precipitate of Ni(CN)2, which does not further release its cyanide
ligands and hence does not dissolve.
On addition of hydrogen peroxide, the precipitate at once is oxidized. In the
fairly strongly acidic conditions of the experiment, the nickel at once forms a
precipitate of Ni(CN)2, which does not further release its cyanide
ligands and hence does not dissolve.
2Zn[Ni(CN)3] + H2O2 + 4H+ → 2Zn2+ + 2Ni(CN)2 + 2HCN + 2H2O
![]()
The information about this nickel(I) complex is from page 314 of the book Lehrbuch der Anorganischen Chemie, Band II, 7. Auflage, written by Dr. H. Remy and published in 1954. Newer information about this complex reveals that it is not simply Ni(CN)32-, but a dimer with a nickel-nickel bond, having structure [(CN)3Ni-Ni(CN)3]4-. Using this information, the last equation better can be written as
Zn2[(CN)3Ni-Ni(CN)3] + H2O2 + 4H+ → 2Zn2+ + 2Ni(CN)2 + 2HCN + 2H2O
Already at the start of the 20th century, this nickel(I) complex was known and the potassium salt was isolated by Bellucci in 1914 and from detailed and laborious element analysis he correctly determined the empirical stoichiometry K2Ni(CN)3, but he did not known about the dimeric structure of this ion.