


Violent reaction between periodate and hydrazine
Periodate is a strong oxidizer and hydrazine is a strong reductor. If these two are brought in contact with each other, then an extremely violent reaction occurs. Even when aqueous solutions are mixed, the heat of reaction is so high, that iodine, which is formed in the reaction, to a large extent escapes as gas! This is a spectacular demonstration, not useful for synthetic purposes, but rather nice to watch.
![]() When performed as directed below, this experiment is not that dangerous, despite
the violent nature of this reaction. However, it is important to
never scale up the experiment for added effect!
When performed as directed below, this experiment is not that dangerous, despite
the violent nature of this reaction. However, it is important to
never scale up the experiment for added effect!
![]()
![]() Required
chemicals:
Required
chemicals:
-
periodic acid (potassium periodate also works, but effect is somewhat less violent)
-
hydrazine or one of its salts, e.g. hydrazine dihydrochloride
-
when one of the salts of hydrazine is used, then sodium hydroxide is needed as well
![]() Required
equipment:
Required
equipment:
- test tubes
![]() Safety:
Safety:
- Hydrazine and its salts are toxic. Hydrazine is a carcinogen.
- Periodic acid is a very strong oxidizer and is corrosive.
- Sodium hydroxide is very corrosive for the skin and the eyes. Especially contact with the eyes is very dangerous, it easily can blind you. Wear goggles!
- The plume of iodine, formed in this experiment, is corrosive and one should really avoid inhalation of iodine vapor!
![]() Disposal:
Disposal:
- After the reaction, the test tube will contain a lot of crystalline iodine and iodine, dispersed in water. Do not rinse this away. Add some sodium hydroxide to the test tube and rinse well, until all iodine has dissolved. The resulting clear solution can be flushed down the drain with a lot of water.
![]()
Procedure for performing the experiment
![]() If you
have hydrazine or hydrazine hydrate, simply mix this with some water, such that
an appr. 20% solution is obtained. If you don't have this, but you do have
hydrazine dihydrochloride or hydrazine sulfate, then take a spatula full of this
(a few hundreds of mg) and add a 40% solution of
sodium hydroxide to this, until all of the solid has dissolved.
If you
have hydrazine or hydrazine hydrate, simply mix this with some water, such that
an appr. 20% solution is obtained. If you don't have this, but you do have
hydrazine dihydrochloride or hydrazine sulfate, then take a spatula full of this
(a few hundreds of mg) and add a 40% solution of
sodium hydroxide to this, until all of the solid has dissolved.
![]() Take a
spatula full of periodic acid and dissolve this in a small amount of water, such
that it just dissolves.
Take a
spatula full of periodic acid and dissolve this in a small amount of water, such
that it just dissolves.
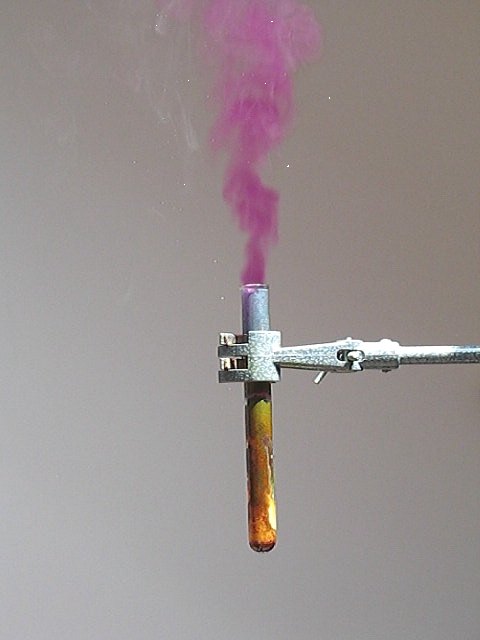
![]() Pour the concentrated solution of periodic acid in the
hydrazine solution. The result is a violent reaction. A plume of iodine vapor
escapes from the test tube and when sunlight is shining on the plume of iodine
vapor, one can see many glittering crystals of iodine, which are formed in the
air, when the vapor cools down again.
Pour the concentrated solution of periodic acid in the
hydrazine solution. The result is a violent reaction. A plume of iodine vapor
escapes from the test tube and when sunlight is shining on the plume of iodine
vapor, one can see many glittering crystals of iodine, which are formed in the
air, when the vapor cools down again.
A video of this reaction is available for download. File size is 1.4 MByte.
Remark: If you don't have periodic acid, but you do have potassium periodate, then a similar effect is obtained by throwing some solid potassium periodate in a concentrated solution of hydrazine dihydrochloride (without the added sodium hydroxide).
![]()
Even more violent reaction
Another, even more violent version of this reaction can be carried out by using solid periodic acid. The preparation of the solution of hydrazine must be done as described above. To this, a pea-sized chunk of solid periodic acid must be added. The result is an extremely violent reaction, in which dense purple clouds of iodine are spewed out of the test tube. Again, a video is avialable for download. File size is 862 kByte.
![]()
Discussion of results
![]() When periodic acid and hydrazine are mixed, then a redox reaction occurs, in
which the hydrazine is oxidized to nitrogen and the periodic acid is reduced to
iodine.
When periodic acid and hydrazine are mixed, then a redox reaction occurs, in
which the hydrazine is oxidized to nitrogen and the periodic acid is reduced to
iodine.
7N2H4 + 4H5IO6 → 7N2 + 2I2 + 24H2O
The above reaction is the main reaction. If a large excess of hydrazine is used, then the iodine is reduced further to hydroiodic acid and the hydrazine forms nitrogen gas.
![]() Another option is to use solid potassium periodate and a solution of hydrazine
dihydrochloride. If this reaction is carried out, then a very similar reaction
occurs. A mix of potassium chloride and hydrochloric acid remains in solution,
but the main redox reaction is very similar.
Another option is to use solid potassium periodate and a solution of hydrazine
dihydrochloride. If this reaction is carried out, then a very similar reaction
occurs. A mix of potassium chloride and hydrochloric acid remains in solution,
but the main redox reaction is very similar.
Many variations are possible:
- solid periodic acid with a hydrazine salt
- solid periodic acid with free base hydrazine
- solid potassium periodate with a hydrazine salt
- solid potassium periodate with free base hydrazine
All these variations give a violent reaction, but most violent is the reaction between the free acid and the free hydrazine base.
Remark: The reaction also works with iodates instead of periodates. E.g. a solution of hydrazine dihydrochloride reacts with solid potassium iodate and this reaction still is very violent.