


Mercury iodide, color change and complex formation
Mercury (II) ions and iodide ions, when brought together form a beautiful orange/red precipitate. This is a well known reaction. But there is more to be said about this simple reaction. Immediately after formation, mercury iodide is not red, but bright yellow. The color of the yellow solid, however, quickly changes to red. Another special property of mercury (II) is that it easily forms a colorless (or very pale yellow) complex with iodide. The bright orange/red precipitate of mercury (II) iodide redissolves again, when more iodide ion is added.
 Mercury
salts are extremely toxic. At any cost, be sure not to be exposed to the mercury
salts, used in this experiment. The orange/red precipitate, although looking
beautifully, also is extremely toxic. Mercury has the additional property that
it is a cumulative poison. Repeated low-level exposure can be as bad as a single
high-level exposure. The mercury builds up in the body and that makes
experimenting with mercury extra riskful.
Mercury
salts are extremely toxic. At any cost, be sure not to be exposed to the mercury
salts, used in this experiment. The orange/red precipitate, although looking
beautifully, also is extremely toxic. Mercury has the additional property that
it is a cumulative poison. Repeated low-level exposure can be as bad as a single
high-level exposure. The mercury builds up in the body and that makes
experimenting with mercury extra riskful.
![]()
![]() Required
chemicals:
Required
chemicals:
-
mercury (II) chloride or mercury (II) nitrate
-
potassium iodide or sodium iodide
![]() Required
equipment:
Required
equipment:
- petri-dish
- test tube
![]() Safety:
Safety:
- mercury (II) salts are extremely toxic (see above)
![]() Disposal:
Disposal:
- The waste MUST be brought to a proper waste processing facility. Mercury salts never may be flushed down the drain.
![]()
Moving band of mercury (II) iodide
![]() Take a
petri dish with a diameter of approximately 8 cm and pour a layer of distilled
water in it, such that it is filled with a layer of 3 to 4 mm of water.
Take a
petri dish with a diameter of approximately 8 cm and pour a layer of distilled
water in it, such that it is filled with a layer of 3 to 4 mm of water.
![]() At one side of the petri dish, near the rim, carefully put a
small amount of solid mercury (II) chloride or mercury (II) nitrate. Try to
avoid motion of the water.
At one side of the petri dish, near the rim, carefully put a
small amount of solid mercury (II) chloride or mercury (II) nitrate. Try to
avoid motion of the water.
![]() Quickly
but carefully put a similar amount of potassium iodide or sodium iodide on the
opposite side of the petri dish. Again, try to avoid motion of the water.
It is important that first the mercury salt is added, and
then the iodide.
Quickly
but carefully put a similar amount of potassium iodide or sodium iodide on the
opposite side of the petri dish. Again, try to avoid motion of the water.
It is important that first the mercury salt is added, and
then the iodide.
Initially, the situation is as follows, with the piece of mercury (II) chloride at the left and the piece of potassium iodide at the right:

After some time, a nice bright orange band of mercury iodide is formed. The band moves towards the mercury chloride. At the front, mercury iodide is formed, and at the back (at the side of the potassium iodide), the band redissolves again.
After a few minutes, the situation is as follows, with the potassium iodide completely dissolved, and the mercury chloride still present as solid:
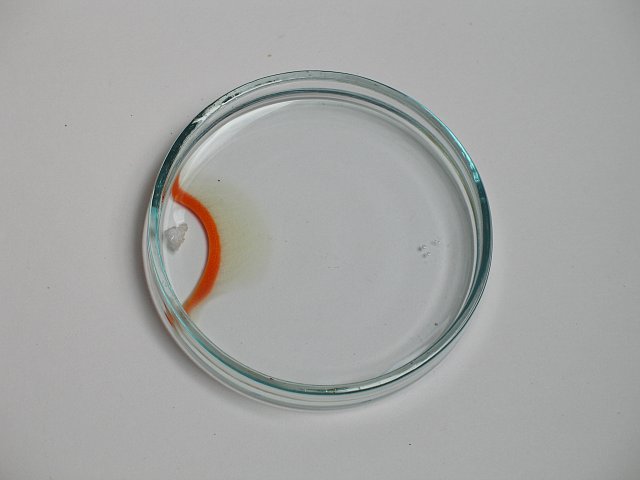
This experiment was done, with a camera present, which made a picture every 15 seconds. These pictures are combined in a small animation, with each frame being displayed for 1 second.
The real speed of the reaction is 1/15 of the speed of the animation.
![]()
Color change of mercury (II) iodide
In this experiment, a small amount of a solution of potassium iodide is put in a petri dish and a small excess amount of a solution of mercury (II) iodide is poured into the petri dish with solution of potassium iodide. This results in formation of a yellow precipitate, which very quickly turns orange/red.




Click here for a nice movie of this reaction. The file size is just over 2 MByte, download speed depends on your internet connection. At the end of this reaction, the entire petri dish is filled with a beautiful bright orange/red precipitate. When excess potassium iodide is added to this, then the precipitate redissolves again, leaving a colorless liquid. A movie of that reaction can be viewed by clicking here. The file size for this is just under 2 MByte.
![]()
Discussion of results
Mercury (II) ions and iodide ions give rise to formation of a highly insoluble compound, HgI2.
Hg2+(aq) + 2I–(aq) → HgI2(s)
Initially, the color of HgI2 is yellow, but the color quickly changes to orange/red. The mechanism behind this color change is not clear. Only the red form is stable, the yellow form is a transient species.
When much more iodide ions are added, then the precipitate quickly redissolves again:
HgI2(s) + 2I–(aq) → HgI42-(aq)
The ion HgI42- is colorless. When viewed very carefully, then this solution of HgI42- is very pale yellow. Whether this is due to the color of this ion, or due to slight oxidation of iodide to iodine is not clear.