


Explosive mix with peroxo chromate
It is well known that some chemical compounds have explosive properties, and that even unconfined small amounts of these compounds explode (or even detonate), when ignited. An example of such a compound is silver acetylide. In this experiment, however, a mix of two compounds is demonstrated, which has equally strong explosive properties as silver acetylide. The mix was prepared without special equipment, just crushing of crystals and carefully mixing by swirling around the two chemicals in a small aluminium dish. Quantities in the order of 10 to 20 mg give an impressive explosion!
Also a mix with red phosphorus and some fine aluminium powder was tried. This mix burns much brighter, but the explosion is not nearly as impressive. The high pitched "bang" is replaced by a lower "whomp" sound.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium tetraperoxo chromate (V)
-
red phosphorus
-
aluminium powder
![]() Required
equipment:
Required
equipment:
-
small aluminium dish
-
miniature spatula
![]() Safety:
Safety:
-
 The mix, prepared
in this experiment is strongly explosive, and it is friction sensitive. When
preparing the mix, use very small quantities, and do not apply any friction
on the mix of chemicals. Crush crystals in separate small dishes and
then mix the two chemicals by carefully swirling around. Do not mix by means
of a spatula or a stick.
The mix, prepared
in this experiment is strongly explosive, and it is friction sensitive. When
preparing the mix, use very small quantities, and do not apply any friction
on the mix of chemicals. Crush crystals in separate small dishes and
then mix the two chemicals by carefully swirling around. Do not mix by means
of a spatula or a stick. -
Red phosphorus is very flammable.
-
Potassium tetraperoxo chromate(V) is a strong oxidizer.
-
Potassium tetraperoxo chromate(V), albeit a chromium (V) compound, most likely has the same or worse toxic effects as chromium (VI) compounds. Treat it as a carcinogen, so avoid breathing any dust or smoke from the experiments.
-
Perform the experiment outside, or use adequate ventilation. If the mix is low in red phosphorus, then the excess potassium tetraperoxochromate(V) will decompose, one of the decomposition products being potassium chromate, which goes into the air as one of the smoke constituents. The risk is not that high, due to the very small amounts of chemicals used, but it is better to be safe than sorrow.
![]() Disposal:
Disposal:
-
A small amount of smoke is produced, but no solid waste.
Remark: Potassium tetraperoxochromate(V) is not a commercially available chemical, but it can easily be prepared from common reagents. How it can be prepared is described on this page.
![]()
Preparation of the mix
![]() Take 50 mg of potassium tetraperoxo chromate(V). Put the
crystals in a small aluminium dish and crush them with a miniature metal
spatula, until a fine dirty brown powder is obtained.
Take 50 mg of potassium tetraperoxo chromate(V). Put the
crystals in a small aluminium dish and crush them with a miniature metal
spatula, until a fine dirty brown powder is obtained.
![]() For
optimal explosive properties take 10 mg of finely powdered red phosphorus.
A slight excess may be used, in order to assure that all
chromium is reduced to the +3 oxidation state (which is much better, due to the
toxic properties of hexavalent and probably also pentavalent chromium).
For
optimal explosive properties take 10 mg of finely powdered red phosphorus.
A slight excess may be used, in order to assure that all
chromium is reduced to the +3 oxidation state (which is much better, due to the
toxic properties of hexavalent and probably also pentavalent chromium).
![]() Add the two powders to each other and carefully swirl the
aluminium dish, such that the powders are mixed.
Add the two powders to each other and carefully swirl the
aluminium dish, such that the powders are mixed. ![]()
![]() Do not mix them by
using a stick, or by means of rubbing. The mix is very friction sensitive. One
time, I had a premature ignition, simply by mixing carefully with a small
spatula. Be prepared for such ignitions, so also do not scale up the experiment.
Be careful!!
Do not mix them by
using a stick, or by means of rubbing. The mix is very friction sensitive. One
time, I had a premature ignition, simply by mixing carefully with a small
spatula. Be prepared for such ignitions, so also do not scale up the experiment.
Be careful!!

![]()
Igniting the mix
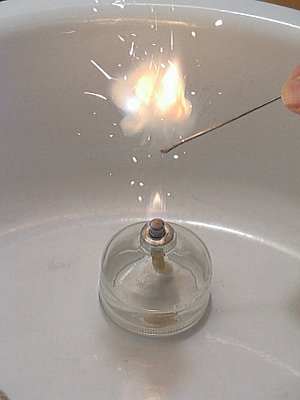
For igniting of the mix, a very small metal spatula was used. Only quantities of 10 to 20 mg were ignited. Below, a picture of the spatula is shown. The picture demonstrates the small size of it.

In order to get an impression of the tiny amounts of mix, used in this experiment, have a look at the following picture. This picture shows a close up of 20 mg of the mix. This small amount gives a really loud bang.

The mix explodes with a loud report, but the amount of light, emitted during the explosion is not very high. The picture below shows the moment of explosion of 20 mg of the mix.

In order to get an impression of the sound of the explosion, play this little movie. It nicely demonstrates the loud bang of the explosion. The movie has a size of approximately 1.5 MByte, download speed depends on your internet connection.
![]()
The effect of adding some aluminium powder
When aluminium powder is added to the mix, at the expense of some red phosphorus, then the mix still is very easy to ignite and also it still is as friction sensitive as the mix without aluminium. It burns with a much larger light output, but the explosion of the unconfined powder is less impressive. An experiment was done by mixing 50 mg of the potassium peroxo chromate (V), 8 mg of red phosphorus, and 7 mg of aluminium powder (particle size 40 Ám). Igniting 20 mg of this mix has the following result:

The left image shows that the light output is so much that the camera is totally wreaking havoc on this. It is totally over exposed. The right image shows the tail of the fast burning.
Click here for a small movie, which demonstrates the sound of the burning mix.
![]()
A reference experiment, mixing red P with K2Cr2O7
As a reference experiment, the burning properties of a mix of red phosphorus and potassium dichromate was tested. For this, 50 mg of potassium dichromate was mixed with 7 mg of red phosphorus (reflecting total reduction of all hexavalent chromium to trivalent chromium with a 10% excess of red phosphorus). This mix, however, only is smouldering a little bit and if it really burns, then it just is due to the burning of the red phosphorus. So, potassium dichromate does not make an energetic mix as potassium tetraperoxo chromate (V).
![]()
Discussion of the results
The tetraperoxo chromate(V) ion is very rich in oxygen, which is easily given off. Red phosphorus on the other hand is extremely flammable, and the combination of these two, when brought together, makes a very powerful explosive.
The redox part of the reaction can be described as follows:
2K3CrO8 + 4P → 3K2O + P4O10 + Cr2O3 + a lot of energy
Of course, in reality, the K2O and P4O10 will combine as potassium phosphate and the chromium (III) also can form chromites. The equation only shows the redox properties. The chromium goes to the +3 oxidation state, and the phosphorus to the +5 oxidation state. All oxygen goes to the -2 oxidation state.
When aluminium is added as well, then that is oxidized to Al2O3, and in that reaction a large amount of light is produced, because of the very high temperature involved in that reaction.
It is remarkable to see that no gaseous products are produced in the reaction, and still there is such a strong explosive power.