


Cyanate ion: uncommon but
interesting ion
This web page gives a series of experiments, which all can be performed easily and safely. Not all experiments need to be done. If one of the required compounds is not available, then other experiments on this page still can be done if those reagents are available.
![]()
![]() Required chemicals:
Required chemicals:
- sodium cyanate or potassium cyanate
- moderately concentrated nitric acid
(35% or so)
- moderately concentrated sulfuric acid (30% or so)
- cobalt sulfate
- silver nitrate
- vanadyl sulfate
- chrome alum or normal (purple) chromium(III) sulfate
- ferric ammonium sulfate
- copper sulfate
- nickel sulfate
![]() Required equipment:
Required equipment:
- test tubes
- small beaker
- many plastic spatulas
![]() Safety:
Safety:
- Nitric acid of moderate concentration is quite corrosive. Avoid contact with skin and eyes.
- Sulfuric acid of moderate concentration is quite corrosive. Avoid contact with skin and eyes.
- Vanadyl sulfate is fairly toxic.
Avoid exposure.
- Cobalt sulfate is toxic. Avoid
exposure.
- Nickel sulfate is toxic and
possibly carcinogenic. Avoid exposure.
- Silver nitrate is caustic and
causes black stains on the skin. Avoid contact,
particularly contact with the eyes must be avoided!
![]() Disposal:
Disposal:
- Waste of vanadium, cobalt, nickel,
silver and copper should not be flushed down the
drain. Bring waste of these metals as heavy metal
waste to a municipal waste processing facility.
Sodium cyanate is non-toxic,
despite it being derived from cyanide ion. In acidic
conditions (such as exist in the stomach) it is
converted to ammonium ions and carbon dioxide in a
matter of minutes.
![]()
Preparation of a solution of sodium
cyanate
If you perform a row of different
experiments with a solution of sodium cyanate, then it
is easiest to prepare a little stock (appr. 30 ml of
solution) in advance. This is enough for all experiments
on this web page. Below follows a picture of some solid
NaOCN in a test tube. The solid is a white crystalline
powder, not looking particularly spectacular.

I had a one-time opportunity to buy a
nice amount of this chemical for just a few euros. Here
follows a picture of the old bottle, which still is in
very good condition.

![]() Put the
approximately 2 grams of solid sodium cyanate in a small
beaker.
Put the
approximately 2 grams of solid sodium cyanate in a small
beaker.
![]() Add
approximately 30 ml of cold distilled water to the
solid.
Add
approximately 30 ml of cold distilled water to the
solid.
![]() Using
a glass rod, stir the liquid, until all of the solid has
dissolved. This takes some time. Sodium cyanate does
dissolve fairly well in water, but it does so slowly.
Using
a glass rod, stir the liquid, until all of the solid has
dissolved. This takes some time. Sodium cyanate does
dissolve fairly well in water, but it does so slowly.
The resulting liquid is clear and
colorless, having a concentration of nearly 7% by weight
of sodium cyanate. For each of the experiments, 4 to 5
ml of solution is used in test tubes.
![]()
Cyanate ion reacts with strong acids, giving mainly isocyanic acid, O=C=N-H. This tautomerizes to cyanic acid H-O-C≡N in an equilibrium reaction. Approximately 97% of the acid exists as isocyanic acid and approximately 3% exists as cyanic acid, when in aqueous solution. The cyanic acid is unstable and quickly hydrolyzes in the acidic solution to give ammonium ions and carbonic acid. The latter decomposes to water and carbon dioxide.
In this experiment, the reaction between sodium cyanate and an excess amount of strong acid is demonstrated. Most interesting is the observation that the formation of carbon dioxide is not instantaneous. This is in strong contrast to adding an excess amount of strong acid to a solution of sodium carbonate or sodium bicarbonate, where the liquid immediately foams up strongly and all carbonate is decomposed at once after adding the acid.
With the cyanate, the liquid at first does not seem to react at all. After a few seconds, the liquid starts bubbling and slowly the intensity of the bubbling increases. The liquid also heats up a little. The 7% solution of sodium cyanate becomes quite warm (but not hot), a few tens of seconds after adding the acid.

In this video one can nicely see the delayed formation of carbon dioxide on addition an excess amount of 30% sulfuric acid.
![]()
In this experiment, it is shown that silver(I) cyanate is insoluble in water. This is a simple experiment.
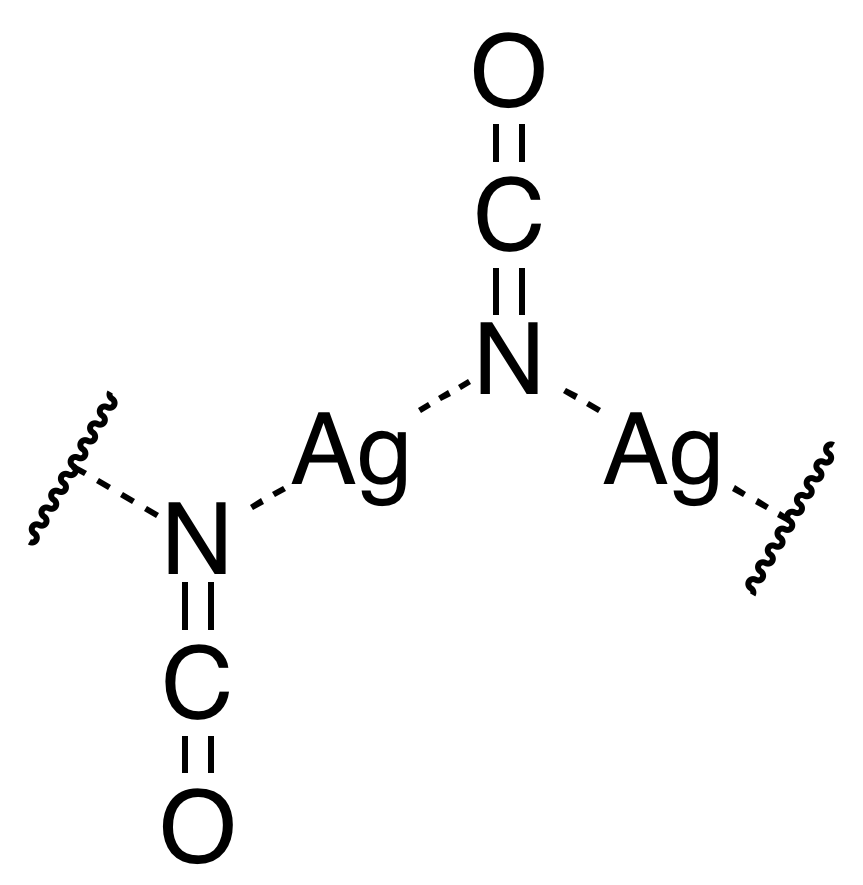
The result is formation of a very pale beige (nearly white) precipitate of silver(I) cyanate. Click here for a video of the reaction. The pale beige solid, formed in the reaction, is not a true salt, but a polymeric species, with a structure as shown in the picture below. See also the Wikipedia page about this compound.
![]()
In this experiment, an interesting deep blue cobalt complex is formed. This complex is quite labile. It is demonstrated that it is in equlibrium with hydrated cobalt(II) ions and when the cyanate ion is taken away, then the complex quickly falls apart again.
![]() Put a few ml
of the 7% solution of sodium cyanate in a test
tube.
Put a few ml
of the 7% solution of sodium cyanate in a test
tube.
![]() In
another test tube, prepare a solution of
cobalt sulfate. A small spatula of the
solid in a few ml is sufficient for this
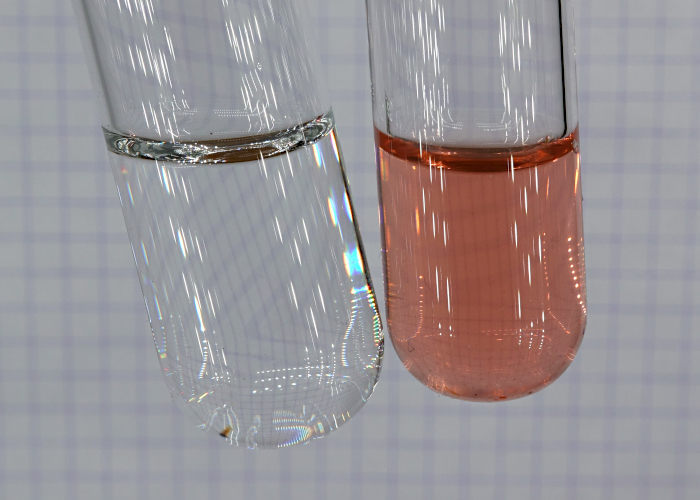
experiment. The solution, thus obtained,
is pink/rose.
In
another test tube, prepare a solution of
cobalt sulfate. A small spatula of the
solid in a few ml is sufficient for this
experiment. The solution, thus obtained,
is pink/rose.
Below, a picture is shown of
the two solutions:
When these two solutions are mixed, then a bright
blue solution is obtained. A video is available of
this reaction. The picture below shows the final
result of the reaction.

This blue complex is quite
specific for the combination of cobalt(II) and
cyanate. It exists at low concentration and is
different from many other blue cobalt complexes,
which require a high concentration of the ligand
(e.g. for formation of the blue tetrachloro
complex of cobalt(II) one needs a very
concentrated solution of NaCl or quite
concentrated HCl).
The complex also can exist
at low concentrations, which means that the
ligands are fairly strongly attracted by the
cobalt(II) core of the complex ion. On the other
hand, the complex is sufficiently labile to allow
for sufficient free cyanate ions, which can be
taken away from the solution very quickly. This is
demonstrated in two ways:
- Addition
of excess amount of silver(I) ions, which leads
to precipitation
of AgOCN.
- Addition of an excess amount of a strong acid, which leads to immediate formation of O=C=N-H. The protonated ion cannot coordinate to the cobalt metal ion and hence the blue color disappears again and the pink color of aqueous cobalt(II) appears again.
Click the above links for videos of these reactions.
The picture below shows the
result of adding silver nitrate to the blue
complex, after allowing the precipitate to settle
somewhat at the bottom.

This picture nicely shows
the pale beige color of the precipitate of AgOCN,
the pink color of the free aqueous cobalt(II) ions
and some blue liquid, which did not mix with the
main part of the solution.
![]()
In the above experiment, the
cobalt(II) complex of cyanate ion is prepared and
some properties are demonstrated. Other metal
ions, however, also form complexes with cyanate
ion and some of them are quite interesting. All of
the prepared complexes are quite labile, just like
the cobalt(II) complex.
![]() First,
metal salts are dissolved in distilled water, in
different test tubes, which were put in a glass
container in order to be able to make pictures of
the solutions.
First,
metal salts are dissolved in distilled water, in
different test tubes, which were put in a glass
container in order to be able to make pictures of
the solutions.

- vanadyl sulfate
- chromium(III) sulfate
- ferric ammonium sulfate
- copper sulfate
- nickel sulfate

The picture shows that in
general, all solutions obtain a more intense
color, while the metal salt is diluted by the
addition of the solution of sodium cyanate. The
color of the chromium complex also shifts from
purple to grayish green.
![]() While
looking at the complexes,
one can see a slow change
of the colors after the
initial immediate color
change. The color of the
chromium complex shifts
towards brighter green and
the color of the iron(III)
complex shifts towards red
and also intensifies. A
new picture was made three minutes
later.
While
looking at the complexes,
one can see a slow change
of the colors after the
initial immediate color
change. The color of the
chromium complex shifts
towards brighter green and
the color of the iron(III)
complex shifts towards red
and also intensifies. A
new picture was made three minutes
later.

The picture
above shows how the colors are
observed under high quality LED light
or under daylight by the eye. On a
digital camera, the color of the
chromium(III) complex looks different
from what you see with the eye:

The last picture
(immediately above) is the picture, as
it comes directly from the digital
camera, but this is definitely not how
it looks to the eye. The previous
picture is how it is perceived by the
eye, but in order to get the image
like that, it was necessary to change
the hue settings for the chromium(III)
complex (the hues for the other
complexes were not adjusted).
The strange behavior in color rendering of the digital camera for the chromium complex is most likely due to the presence of narrow absorption bands in the visible spectrum. Digital photo cameras have difficulty rendering colors correctly, when the spectrum of the light is not spread smoothly, but has narrow bands and peaks.
![]() Finally,
some 30%
sulfuric acid
is added to
each of the
test tubes in
order to see
whether the
complex
remains
present or is
destroyed
immediately.
For each of
the metals,
the complex is
destroyed
immediately
and the
solutions
become pale
again. Several
seconds later,
the liquids
start
bubbling. So,
in a very fast
reaction, the
metal
complexes are
broken down
and the
cyanate-ligands
are protonated
and then in a
subsequent
slower
reaction, the
protonated
cyanate
decomposes,
giving carbon
dioxide.
Finally,
some 30%
sulfuric acid
is added to
each of the
test tubes in
order to see
whether the
complex
remains
present or is
destroyed
immediately.
For each of
the metals,
the complex is
destroyed
immediately
and the
solutions
become pale
again. Several
seconds later,
the liquids
start
bubbling. So,
in a very fast
reaction, the
metal
complexes are
broken down
and the
cyanate-ligands
are protonated
and then in a
subsequent
slower
reaction, the
protonated
cyanate
decomposes,
giving carbon
dioxide.

The iron(III) complex almost completely disappears and becomes colorless. This is due to the excess amount of added acid, which causes the brown partially hydrolyzed iron(III) to be converted to nearly colorless non hydrolyzed aqueous iron(III).
![]()
Discussion of results
Solid sodium cyanate exists in anhydrous form, as NaOCN. When this is dissolved, the solution obtains sodium ions and cyanate ions:NaOCN(s) → Na+(aq) + OCN–(aq)
H+(aq) + OCN–(aq) → O=C=N-H(aq) (this is isocyanic acid)
Isocyanic acid tautomerizes to cyanic acid in an equilibrium reaction. The equilibrium is at 97% at the isocyanic acid side and 3% at the cyanic acid side.
O=C=N-H ⥃ H-O-C≡N
The latter is not stable and reacts with water and excess acid in a somewhat slower reaction with formation of ammonium ions and carbonic acid, which in turn decomposes to water and carbon dioxide:
H-O-C≡N + H+ + 2 H2O → NH4+ + H2CO3 → NH4+ + H2O + CO2
This explains the bubbling after addition of a strong acid to a solution of a cyanate. In practice, cyanate ion cannot exist at low pH for more than a few tens of seconds.
2n Ag+(aq) + 2n OCN–(aq) → [⋯Ag⋯N(=C=O)⋯Ag⋯N(=C=O)⋯]n (s)
The structure of the solid is given below, for one unit of the polymeric species:

The silver ions are bound to two N-atoms, but not with full bonds between the silver atoms and nitrogen atoms.
This compound has very low solubility in water and it also is not easily protonated. On addition of a strong acid, it does dissolve again, but only very slowly. The acid protonates the cyanate-ions, and this leads to breaking of the half-bonds with the silver ions. This causes the chain to break apart and in this way, the solid slowly dissolves. At first, the liquid becomes somewhat opalescent, but after a longer time, when the chains are broken up to very short pieces, the liquid becomes clear.