


Bromide complexes of copper(II)
Copper(II) salts are known to have a sky-blue color in aqueous solution. This blue color is due to formation of the hydrated ion Cu(H2O)42+. The water ligands, however, can easily be exchanged with other ions. It is fairly well known that replacement of water ligands by chloride ions leads to formation of green or even yellow complexes. When bromide is used instead of chloride, then remarkable colors are obtained. With bromide, copper(II) can form deep purple or deep red complexes. Mixing bromide and chloride leads to formation of red/orange complexes.
![]()
![]() Required
chemicals:
Required
chemicals:
-
copper sulfate
-
concentrated hydrobromic acid (40% by weight)
-
concentrated hydrochloric acid (30% by weight)
-
potassium bromide
![]() Required
equipment:
Required
equipment:
- test tubes
![]() Safety:
Safety:
- Concentrated hydrobromic acid and concentrated hydrochloric acid are corrosive and give off irritating corrosive fumes.
![]() Disposal:
Disposal:
- Do not flush the solutions in the sink. Dilute them somewhat with water and keep them until they are brought to a proper chemical waste processing facility.
![]()
Copper(II) complex in concentrated hydrobromic acid
Dissolve some copper sulfate in 40% hydrobromic acid. This leads to formation of a purple solution, which has a very strong color. The picture below shows a solution of only a small amount of copper sulfate (a little heap on the tip of a small screwdriver) in 1 ml of hydrobromic acid. The thin layer of liquid, sticking to the glass already has a strong color, the liquid itself looks almost black.

The color of the complex is deep purple. When chloride ion is added as well, then the color of the solution shifts from deep purple to brown/red, through shades of red. The four pictures below show the liquid, after adding more and more of concentrated hydrochloric acid (30%). After adding just a few drops, the liquid becomes more or less blood-red, adding more hydrochloric acid makes the color shifts towards yellow in thin layers (sticking to the glass) and red when viewed through a thicker layer.




When water is added, then the color of the liquid quickly changes towards blue. The two pictures below show the result of adding water to the red/brown solution, as shown in the last picture above. The picture at the left shows the result of adding water, such that the concentration of the acid-mix drops to approximately 20% by weight. In this situation, the liquid looks yellow/green. When much more water is added, then the liquid becomes very light blue, and all copper is present as simple hydrated ion.


The last picture shows a test tube with an almost colorless solution, but when one is looking through the entire column of liquid, then one can clearly see the blue color of the liquid, still with a slight greenish hue:
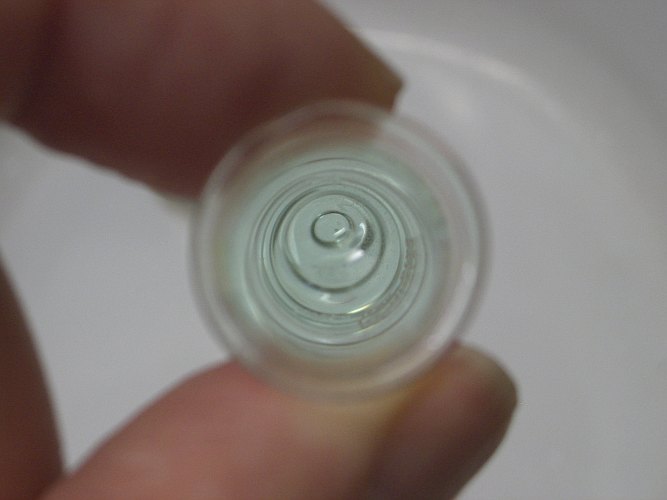
This experiment also nicely demonstrates that the color of the bromide complex is incredibly intense, compared to the color of plain hydrated copper(II) ions. The first picture, showing the deep purple liquid is based on the same amount of copper as the last picture with the pale blue liquid.
![]()
Copper(II) complex in concentrated solution of KBr
When copper(II) sulfate is dissolved in a saturated solution of potassium bromide, then one obtains a deep red/brown solution, but the color of this liquid is not as intense as the color of the solution in 40% HBr. The color also is somewhat different, it is not purple, but brown/red. The picture below shows the solution of copper sulfate in a saturated solution of potassium bromide. Not all of the potassium bromide was dissolve, some solid potassium bromide still sticks to the glass.

Light transmitted through this liquid is red/brown, and the liquid, sticking to the glass only gives a very faint brown color around the crystals of undissolved potassium bromide. The amount of copper sulfate, used in this experiment, is similar to the amount, used in the experiment with HBr. So, in a solution of HBr, the color of the complex is much more intense, and the color also is different. Apparently, the acid has a strong influence on the color.
When the solution is diluted with water, then the color quickly goes to pale blue, through shades of yellow. The three pictures below show the same test tube, each time with a little bit more water added. All remaining solid potassium bromide is dissolved now.



![]()
Discussion of results
![]() Copper ions can form deeply colored complexes with bromide ions. According to
literature, the complex ion CuBr42- is formed at
sufficiently high concentration of bromide ion. At lower concentration of
bromide, ions can be formed with a lower bromide content, such as [CuBr3(H2O)]–.
Copper ions can form deeply colored complexes with bromide ions. According to
literature, the complex ion CuBr42- is formed at
sufficiently high concentration of bromide ion. At lower concentration of
bromide, ions can be formed with a lower bromide content, such as [CuBr3(H2O)]–.
![]() The color of the complex ion in hydrobromic acid
differs from the color of the complex ion in saturated solution of potassium
bromide. Most likely this is due to coordination of H+ as well.
Probably there are complex ions like HCuBr4–.
The color of the complex ion in hydrobromic acid
differs from the color of the complex ion in saturated solution of potassium
bromide. Most likely this is due to coordination of H+ as well.
Probably there are complex ions like HCuBr4–.
![]() In a solution with both HBr and HCl, mixed complexes
are formed, with chloride and bromide in the ion:
CuCl4-xBrx2-. Here, x depends on the ratio of
chloride and bromide. Probably, there also is H+
coordinated as well.
In a solution with both HBr and HCl, mixed complexes
are formed, with chloride and bromide in the ion:
CuCl4-xBrx2-. Here, x depends on the ratio of
chloride and bromide. Probably, there also is H+
coordinated as well.
Remark: If you do not have access to 40% HBr, then this experiment also can be done with a 50% solution of H2SO4, which is saturated with KBr. Such an acid solution produces a differently colored complex than a neutral solution of KBr.