


Cobalt thiocyanato mercurate(II): Beautiful and toxic
The chemistry in this experiment is really amazing. A beautiful deep red solution is prepared and from this solution a very bright blue precipitate is produced. This is most remarkable, seeing the formation of beautiful very bright blue crystals from an equally bright red solution.

![]()
![]() Required
chemicals:
Required
chemicals:
-
mercury(II) chloride
-
cobaltous chloride (the sulfate or nitrate can also be used)
-
concentrated hydrochloric acid (30% HCl by weight)
-
ammonium thiocyanate
![]() Required
equipment:
Required
equipment:
- test tubes
![]() Safety:
Safety:
- Mercury(II) chloride is very very toxic. Avoid contact with this chemical! It is a very potent and cumulative poison.
- Concentrated hydrochloric acid is corrosive and gives off nasty fumes.
- Salts of cobalt are quite toxic as well.
![]() Disposal:
Disposal:
- Keep the waste of this experiment and bring it as heavy metal waste to a municipal waste processing facility. Do NOT flush the waste down the drain.
![]()
Preparation of remarkable complex
In the following steps it is described how the bright blue complex can be produced, which precipitates from a bright red solution.
![]() Dissolve a small spatula of mercury(II) chloride in a few ml
of concentrated hydrochloric acid. The solid easily dissolves in the strong
acid, much easier than it does in plain water. This solution is colorless.
Dissolve a small spatula of mercury(II) chloride in a few ml
of concentrated hydrochloric acid. The solid easily dissolves in the strong
acid, much easier than it does in plain water. This solution is colorless.
![]() Dissolve
some cobaltous nitrate or cobaltous chloride in concentrated hydrochloric acid.
This produces a deep blue solution.
Dissolve
some cobaltous nitrate or cobaltous chloride in concentrated hydrochloric acid.
This produces a deep blue solution.
![]() Mix both
solutions. This gives a clear deep blue solution. No precipitate is formed.
Mix both
solutions. This gives a clear deep blue solution. No precipitate is formed.
![]() In a separate test tube dissolve a large spatula full (excess
amount, relative to the cobalt and mercury salts) of ammonium thiocyanate. This
is a colorless solution. This solution must have a volume, which is appr. half
the volume of the acidic cobalt/mercury chloride solution.
In a separate test tube dissolve a large spatula full (excess
amount, relative to the cobalt and mercury salts) of ammonium thiocyanate. This
is a colorless solution. This solution must have a volume, which is appr. half
the volume of the acidic cobalt/mercury chloride solution.
![]() Mix the contents of both solutions and swirl, such that the
liquids mix well. The color of the resulting liquid becomes deep red and remains
clear. This change from deep blue to deep red already is quite special.
Mix the contents of both solutions and swirl, such that the
liquids mix well. The color of the resulting liquid becomes deep red and remains
clear. This change from deep blue to deep red already is quite special.
After a few minutes, however, small bright blue crystals are formed in the deep red solution and these slowly fall to the bottom, like snow.

The liquid keeps on producing little blue crystals for a few minutes. After 5 minutes or so, the liquid is somewhat lighter and production of small crystals ceases. At that time there is a thin layer of blue solid on the bottom.
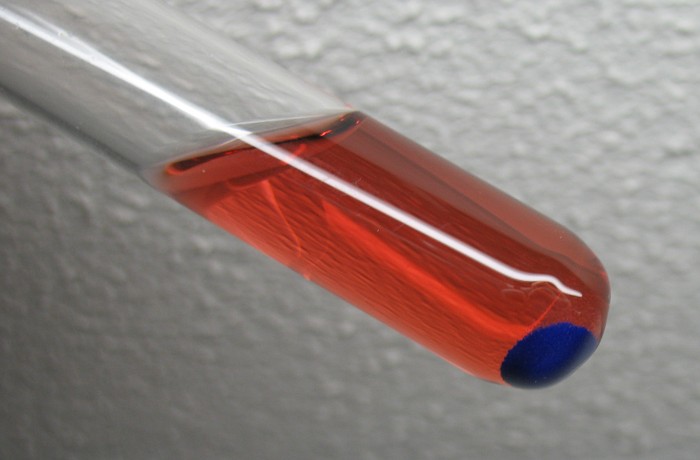
After a period of 24 hours, the liquid still is red, but the color then is much lighter and tends towards pink and orange. A beautiful bright blue precipitate then is present at the bottom of the test tube. The picture below shows the test tube photographed from below, while it was kept nearly horizontally.

Another view of the test tube is shown below, photographed from side/below:
![]()
Repeating of experiment, but in neutral solution instead of 30% HCl
A similar experiment is done in neutral solution.
![]() Dissolve a small spatula of mercury(II) chloride in a few ml
of water. The solid dissolves with difficulty. A saturated solution was prepared
with a little solid mercury(II) chloride still sitting at the bottom.
Dissolve a small spatula of mercury(II) chloride in a few ml
of water. The solid dissolves with difficulty. A saturated solution was prepared
with a little solid mercury(II) chloride still sitting at the bottom.
![]() Add an
excess amount of solid ammonium thiocyanate to the solution of mercury(II)
chloride. The solid quickly dissolves and the remaining mercury(II) chloride now
also quickly dissolves.
Add an
excess amount of solid ammonium thiocyanate to the solution of mercury(II)
chloride. The solid quickly dissolves and the remaining mercury(II) chloride now
also quickly dissolves.
![]() In a
separate test tube dissolve some cobaltous nitrate and add this solution to the
solution of mercury(II) chloride and ammonium thiocyanate.
In a
separate test tube dissolve some cobaltous nitrate and add this solution to the
solution of mercury(II) chloride and ammonium thiocyanate.
If the solutions are mixed, then at first glance nothing special seems to happen. The pink solution only becomes slightly more intensely colored. This is due to formation of a thiocyanato complex of cobalt(II), which has a somewhat stronger red color than the aquated cobalt(II) ion.
After a few minutes, however, many small crystals are produced, which slowly move to the bottom. The slow formation of crystals lasts much longer than in the experiment with acid and finally, after a few hours, much more precipitate has formed, while the amounts of cobalt and mercury were roughly the same in both experiments.
The precipitate is somewhat hydrophobic, it sticks to the glass and to the surface of the liquid. The liquid itself is only pale pink after a few hours. This is another indication that much more of the cobalt is precipitated from solution. Apparently in strongly acidic solution, or at the strong concentration of chloride in the used solution, one of the consituents of the blue complex (most likely mercury(II)) is much more soluble. The picture below shows the result of this experiment in neutral solution after a few hours:

When the test tube is rotated 180 degrees around its axis, then the precipitate is viewed through the pale pink solution and then it looks purple instead of blue. There also is a nice contrast between the precipitate which stick at the side of the glass which is photographed and the opposite side, which is viewed through the pale pink solution.

Finally, both test tubes with the acidic solution and the neutral solution are placed next to each other in order to show the large difference, both in color of the solution and the amount of precipitate.

![]()
Discussion of results
Mercury(II) ion forms insoluble Hg(SCN)2, but when excess thiocyanate is used, then a colorless complex ion is formed, which can be written as Hg(SCN)42–. The ammonium salt of this ion is soluble in water. This explains why mercury(II) chloride easily dissolves in excess amount of a solution of ammonium thiocyanate.
A similar complex is formed with chloride ion: HgCl42–. This explains why HgCl2 dissolves more easily in concentrated HCl than in plain water.
Cobaltous ion also forms a complex with thiocyanate. At very high concentration of thiocyanate a blue complex Co(SCN)42– is formed. At somewhat lower concentration there is partial exchange of ligands with water and then a red mix of complexes [Co(H2O)m(SCN)n]2–n is formed, with n typically in the range 1 ... 3 and m in the range 1 ... 4 (for higher n there will be lower m and vice versa). The red color of this mix of complexes is more intense than the color of the hexaqua complex of cobalt.
Similar behavior is observed for cobaltous ion, combined with chloride ions. This explains why the solution in concentrated hydrochloric acid is deep blue. The blue complex CoCl42– is formed in such solutions. On mixing with the thiocyanate the chloride is diluted and partial exchange occurs with thiocyanate and water ligands. The resulting solution is deep red, like red wine, which is more purple than red.
When all solutions are mixed, then a complicated mix is formed with the mercury thiocyanate complex and partially aquated cobalt thiocyanate complexes. These complexes are in equilibrium with a bright blue two metal complex which can be written as CoHg(SCN)4. The latter is much less soluble than the other complexes and due to this, there is slow precipitation of the blue complex. The equation for the reaction can be written as:
[Co(H2O)m(SCN)n]2–n + Hg(SCN)42– ↔ CoHg(SCN)4 + n SCN– + m H2O
The following reaction drives the equilibrium to the right: CoHg(SCN)4(aq) → CoHg(SCN)4(s)
When chloride is present as well, then there is a competing equilibrium reaction, in which thiocyanate ligands are replaced by chloride ligands. All of the resulting complexes are soluble and hence do not form a precipitate. This explains why in the presence of HCl much less precipitate is formed. More mercury and cobalt remain in solution and the color of the liquid remains more intense as well due to the higher cobalt content.