


Temperature dependence of chromate/dichromate equilibrium
It is well-known that chromate and
dichromate ions in aqueous solutions can be converted
into each other by adding base or acid. At low pH,
hexavalent chromium exists as orange dichromate and at
high pH, hexavalent chromium exists as yellow chromate.
When a strong base is added to a solution of a
dichromate, then the solution goes from orange to
yellow:
Cr2O72– + 2 OH– →
2 CrO42–
+ H2O
When acid is
added to a solution of chromate, then the solution goes
from yellow to orange:
2 CrO42–
+ 2
H+ →
Cr2O72– + H2O
This reaction can be repeated many times, before the original solution becomes too dilute due to all the added acidic or basic solutions.
Much less
known, however, is that the equilibrium between chromate
and dichromate also depends on temperature. The
pH-range, over which there is a shift from orange
dichromate to yellow chromate, can be shifted by
changing the temperature. This can be nicely
demonstrated by taking a yellow solution, with a pH just
beyond the range where the shift occurs from orange to
yellow and heating the solution. The heating moves that
pH-range towards higher pH and hence the solution comes
in the region, where it becomes orange.
 Be
careful with the chemicals, used in this experiment.
Hexavalent chromium is a known carcinogen on inhalation
and some people can show strong allergic reactions on
exposure to hexavalent chromium solutions.
Be
careful with the chemicals, used in this experiment.
Hexavalent chromium is a known carcinogen on inhalation
and some people can show strong allergic reactions on
exposure to hexavalent chromium solutions.
![]()
![]() Required chemicals:
Required chemicals:
- sodium chromate or potassium
chromate
- ammonium perchlorate or ammonium
sulfate or ammonium nitrate (in fact, any soluble
ammonium salt, with an anion, derived from a strong
acid which cannot be oxidized, is suitable)
- ammonia
If ammonium perchlorate is used, then
use sodium chromate, because perchlorate reacts with
potassium ions, giving a precipitate of solid potassium
perchlorate.
![]() Required equipment:
Required equipment:
- test tubes
- heater
![]() Safety:
Safety:
- Hexavalent chromium salts are toxic. If solutions of this are boiled, then assure that no small droplets of the liquid escape into the air. Hexavalent chromium is a known carcinogen when it is inhaled.
![]() Disposal:
Disposal:
- After the experiment, add excess acid to the yellow chromate solutions.
- Next, add a suitable reductor (ethanol, even better is a sulfite or bisulfite solution), such that all chromium is converted to green chromium(III).
- After this treatment, the waste can be flushed down the drain. The quantities used are very small and chromium(III) is not particularly toxic.
![]()
Preparation of two
solutions for comparison
![]() Take a test tube and put a small spatula
full of sodium chromate in this (or potassium chromate)
and dissolve the solid in appr. 7 ml of water.
Quantities are not critical, 200 mg or so is a good
amount.
Take a test tube and put a small spatula
full of sodium chromate in this (or potassium chromate)
and dissolve the solid in appr. 7 ml of water.
Quantities are not critical, 200 mg or so is a good
amount.
![]() Divide the solution over two test tubes.
Divide the solution over two test tubes.
![]() To one of the test tubes, add a spatula
full of the ammonium salt and dissolve this as well. A
similar quantity as taken for the chromate is suitable,
the precise amount is not critical at all.
To one of the test tubes, add a spatula
full of the ammonium salt and dissolve this as well. A
similar quantity as taken for the chromate is suitable,
the precise amount is not critical at all.
![]() Add a single drop of 5%
ammonia to the test tube, to which an ammonium salt is
added.
Add a single drop of 5%
ammonia to the test tube, to which an ammonium salt is
added.
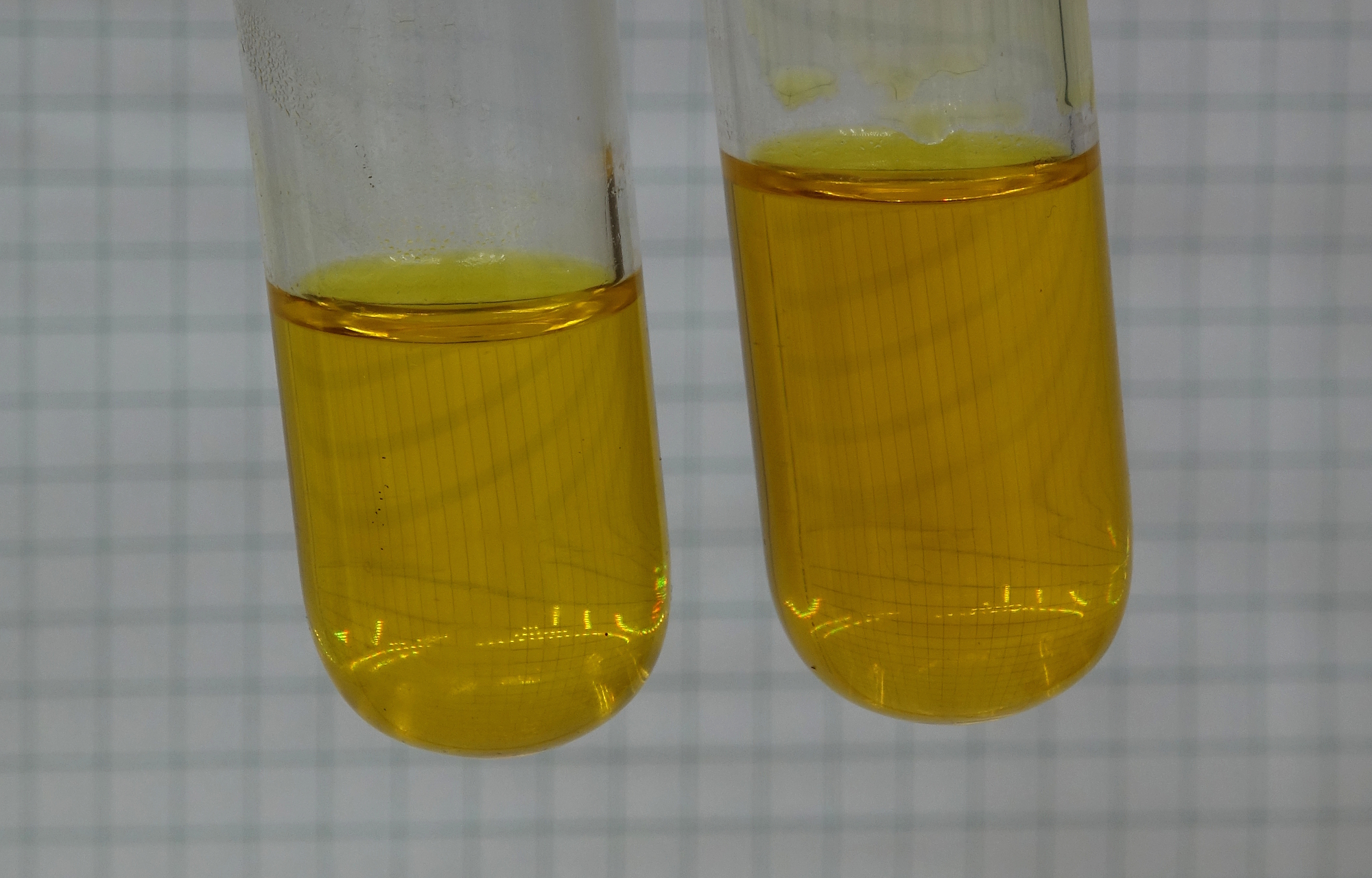
After this, there are two test tubes, each of them holding a yellow solution. These solutions look similar, they cannot be distinguished from each other by color.

The solution at the left only contains the chromate salt, the solution at the right contains the ammonium salt and a single drop of ammonia as well.
The ammonium/ammonia combination acts
as a buffer, keeping the pH fairly constant, even if
some acid is consumed or produced in the solution.
![]()
Heating of both solutions
When the solutions are heated to
near boiling, then the solution in the right test tube
(the one which contains the ammonium salt and ammonia)
turns deep orange, while the solution in the left test
tube remains yellow. In this experiment, first the top
part of the liquid was heated and this results in orange
coloration at the top, while the bottom still is more
yellow.

When the test tubes are swirled on
further heating, then the result is as follows:
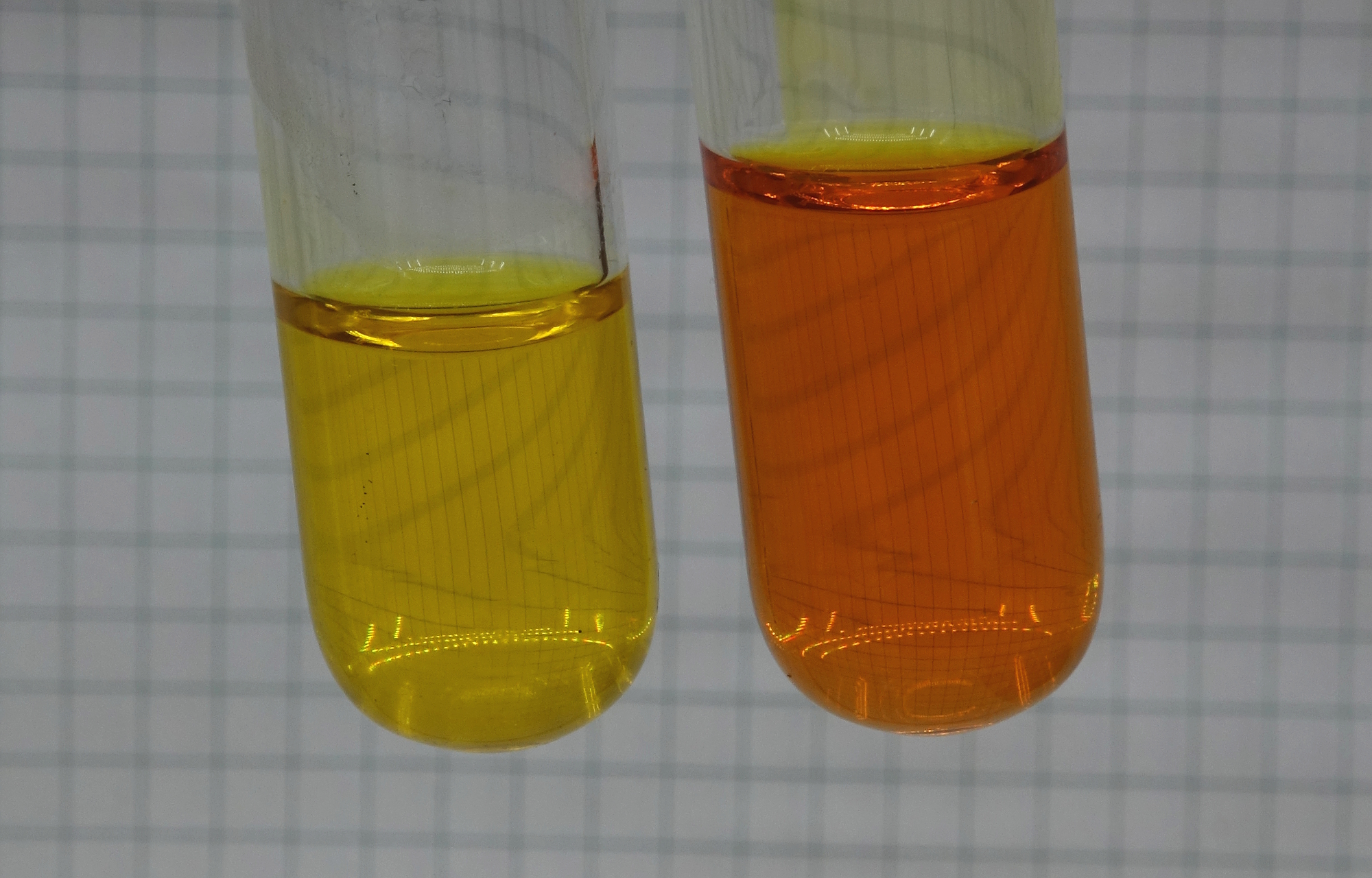
The right test tube now has a deep
orange color. One could think this is due to loss of
ammonia and subsequent lowering of the pH of that
solution, but this is not the case. The loss of ammonia
is kept to a minimum. The liquids were not really
boiled, and during heating, the test tubes were loosely
stoppered with a gag of tissue paper in order to prevent
accidental formation of an aerosol with hexavalent
chromium in it. This, however, was not formed. After the
heating, the gag of tissue paper had no visible yellow
coloring from hexavalent chromium.
![]()
Letting the solutions
cool down again
On cooling down, the liquid in the
left test tube did not change, the liquid in the right
test tube slowly became lighter again.
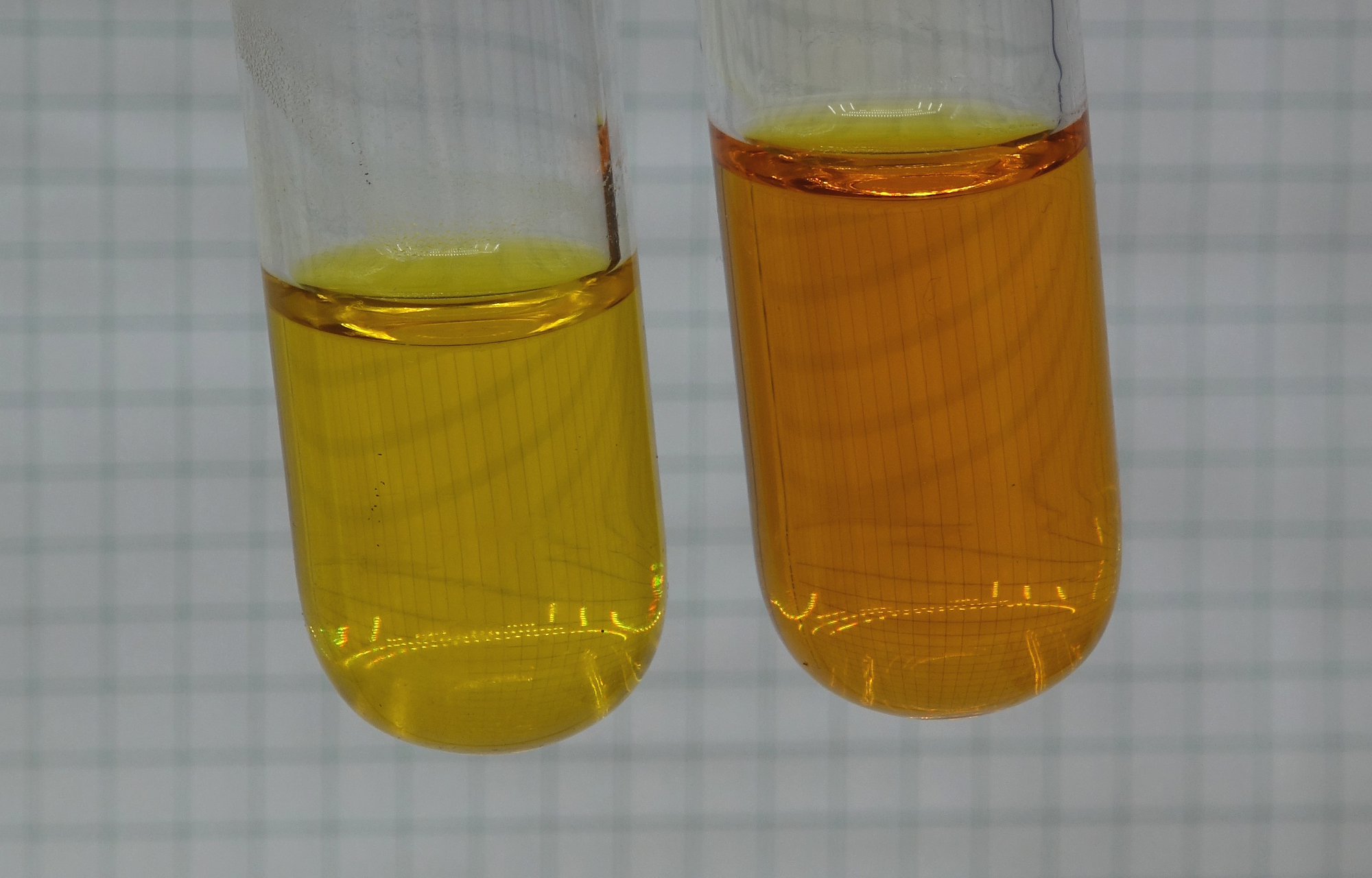


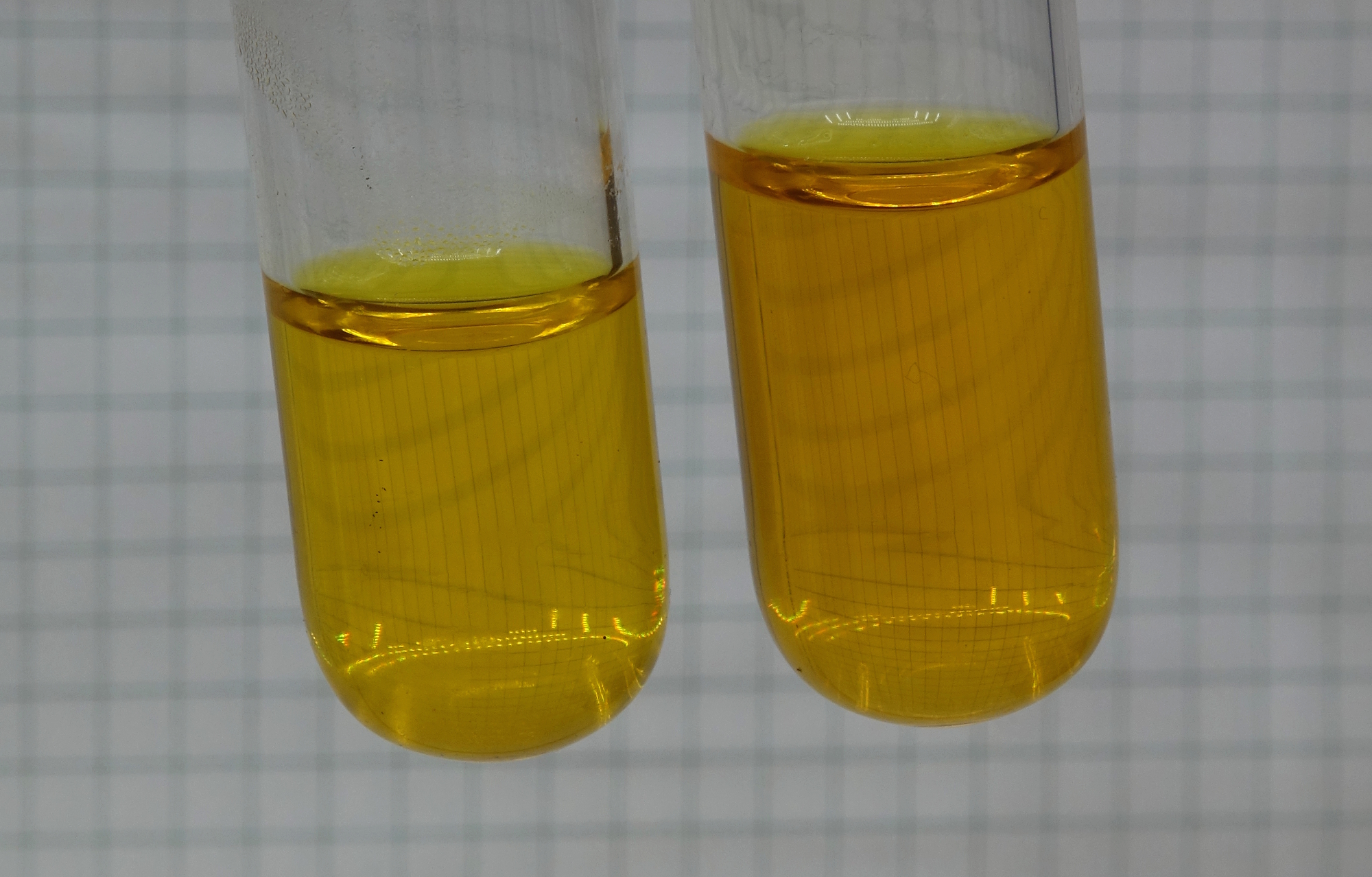
The four pictures, shown above, were taken with intervals of two to three minutes. After approximately 10 minutes, the test tubes still were quite warm.
In order to speed up the process of
cooling down, some liquid butane from a refill canister
for cigarette lighters can be added to the right test
tube. This is shown in the picture below. There is a
thin layer of colorless butane on top of the aqueous
solution. Also some butane was sprayed on the outside of
the test tube.
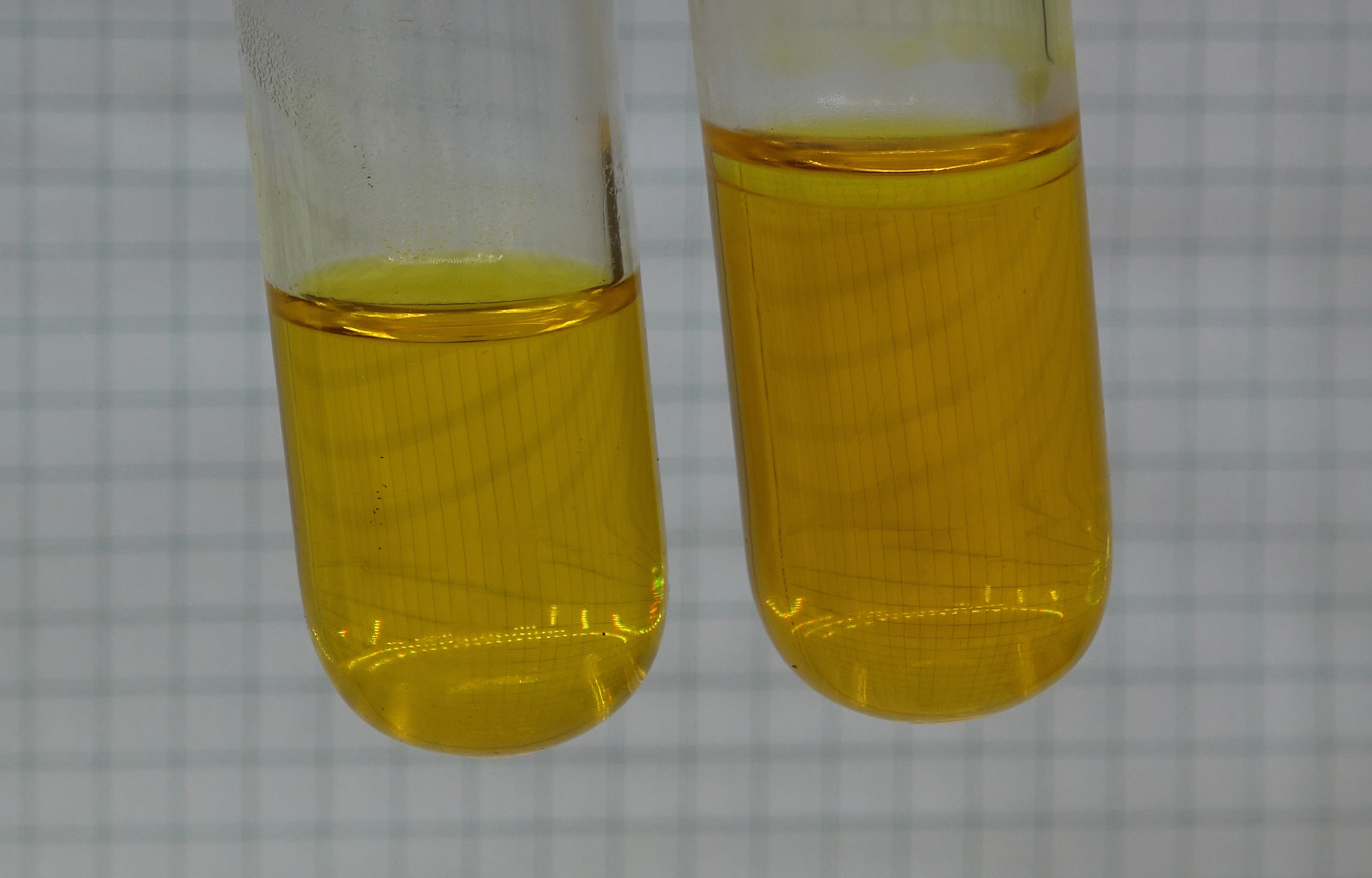
This addition of some butane gas causes the liquid to
cool down much faster (butane has a boiling point of
appr. 0 ºC
and its evaporation takes away quite some heat). When
all butane has evaporated, the liquid in the test tube
is still a little warmer than the ambient temperature,
but the cooling down is sufficient to get its original
yellow color again.
![]()
Discussion of results
![]() The mix of an ammonium salt and a drop of
ammonia makes a crude buffer, which keeps pH fairly
constant on consumption or production of OH– ions or H+ ions.
The mix of an ammonium salt and a drop of
ammonia makes a crude buffer, which keeps pH fairly
constant on consumption or production of OH– ions or H+ ions.
![]() The conversion
from chromate to dichromate can be considered as an
equilibrium reaction:
The conversion
from chromate to dichromate can be considered as an
equilibrium reaction:
2 CrO42– + 2 H+ ↔ Cr2O72– + H2O
This equilibrium apparently is
influenced strongly by temperature. At high temperature
it is more to the right, at low temperature it is more
to the left. The pH, needed to go from orange to yellow
is raised on raising the temperature.