


Red chemiluminiscense
It is very well known that hydrogen peroxide is a strong oxidizer, and under those conditions, it is oxidized to water, or to hydroxide ion. Hydrogen peroxide, however, also can act as a reductor. In that case, it produces oxygen, the oxygen goes from oxidation state -1 to 0. The oxygen, produced in this reaction, however, is not normal oxygen, but so-called singlet-oxygen. It also is O2, but the electronic configuration of this molecule differs from the electronic configuration of ordinary O2.
The singlet-oxygen is very reactive and quickly reacts with almost everything nearby, including other molecules of singlet oxygen. In one of these reactions, a photon of light is emitted, which is visible as red light. This effect is not really strong, but when singlet oxygen is produced at a sufficiently fast rate, then the red glow is clearly visible. This phenomenon is very special and it allows a simple, yet very entertaining experiment, to be conducted.
![]()
![]() Required
chemicals:
Required
chemicals:
-
hydrogen peroxide, 30%
-
swimming pool chlorine (calcium hypochlorite, or trichloroisocyanuric acid, or sodium dichloroisocyanurate, best result is obtained with the last one)
![]() Required
equipment:
Required
equipment:
-
glass beaker or erlenmeyer.
-
test tube
![]() Safety:
Safety:
- Concentrated solutions of hydrogen peroxide are very corrosive for the skin and lead to severe irritations (white spots, which are itching strongly).
- Hypochlorites, chloroisocyanurate, and derivatives of that, are strong oxidizers, which are corrosive to skin and many other materials. Avoid contact with the solids and concentrated solutions.
- During the experiment, irritating and corrosive steam is produced. If the experiment is done in a darkened room, then assure there is very good ventilation, but it is best to do the experiment in a fume hood, or outside, during night time.
![]() Disposal
and cleanup:
Disposal
and cleanup:
- The waste of this experiment may be flushed down the drain. If larger solid particles remain, crunch them with a plastic or glass rod (not a metal rod, unless you don't mind the rod being corroded) and then rinse the waste away with plenty of water (e.g. flushing through the toilet).
![]()
Preparation for the experiment
![]() Put
approximately 1 gram of swimming pool chlorine in a dry beaker or erlenmeyer.
Best results are obtained with Na-DCCA (sodium dichloroisocyanurate). With TCCA
(trichloroisocyanuric acid), the reaction is longer lasting, but quite slow. The
intensity of the red glow is only weak. With calcium hypochlorite, the reaction
is very violent, a little bit too violent.
Put
approximately 1 gram of swimming pool chlorine in a dry beaker or erlenmeyer.
Best results are obtained with Na-DCCA (sodium dichloroisocyanurate). With TCCA
(trichloroisocyanuric acid), the reaction is longer lasting, but quite slow. The
intensity of the red glow is only weak. With calcium hypochlorite, the reaction
is very violent, a little bit too violent.
![]() Put 2 to 3 ml of 30% hydrogen peroxide in a test tube.
Put 2 to 3 ml of 30% hydrogen peroxide in a test tube.
![]()
Performing the experiment
![]() Put the erlenmeyer on a table, in a darkened room or outside
during night time. Best results are obtained at a totally dark place, but a dim
light can be helpful, and makes performing the experiment somewhat safer.
Put the erlenmeyer on a table, in a darkened room or outside
during night time. Best results are obtained at a totally dark place, but a dim
light can be helpful, and makes performing the experiment somewhat safer.
![]() Let the eyes get used to the darkness.
Let the eyes get used to the darkness.
![]() Pour the hydrogen peroxide onto the granulated solid.
Pour the hydrogen peroxide onto the granulated solid.
When the hydrogen peroxide is poured onto the granulated solid, then a nice red glow is produced. In a dimly lit room the result is as follows:


In a totally darkened room, the effect is most spectacular. Of course, in the totally darkened room, one has to be more careful. The chemicals, used in this experiment are not really benign, but with some exercising, the experiment can be conducted safely, also in a completely darkened room. The result of such an experiment is shown here:

![]()
Discussion of results
Three different states of the molecule O2.
Oxygen normally is a colorless gas with molecules, consisting of two atoms, chemical formula O2. Another allotrope of oxygen is the blue gas O3, called ozone. These are well-known. Less known, however, is that three forms exist of the molecule O2. Quantum mechanics and molecular orbital theory is required to precisely describe the nature of these three different forms of oxygen. For the purpose of this page, it is sufficient to say, that the difference is in the occupation of molecular orbitals and in the spin of some of the electrons in the O2 molecule.
For the interested reader, here is some theory, useful for understanding this (requires university level physics and mathematics !):
http://en.wikipedia.org/wiki/Singlet_oxygen
http://www.photobiology.com/educational/len2/singox.html
In short, the three forms of oxygen are denoted as follows:
(1) The most excited state O2(1∑g+). This only lasts for seconds and is converted to (2) a lower, but still excited state O2(1∆g). This lower excited state is fairly stable and lasts for tens of minutes, as long as it is not allowed to react with other molecules. During its decay, it emits infrared radiation at 1269 nm wavelength. Due to its extreme reactivity, in the presence of other molecules at high concentrations, it usually exists for a much shorter time (milliseconds, or even microseconds). The O2(1∆g) molecule decays into (3) normal oxygen, so-called triplet state oxygen, O2(3∑g-).
For the used notation, see here.
Reaction of hydrogen peroxide with hypochlorite
When hydrogen peroxide reacts with hypochlorite, then oxygen is produced, but this oxygen is not normal triplet oxygen, but one of the excited singlet states:
H2O2 + ClO– → H2O + O2(1∆g) + Cl–
Singlet state oxygen is very reactive, and two of these molecules react with each other:
2O2(1∆g) → 2O2(3∑g-) + hν (634 nm)
Radiation at 634 nm is observed as red light. This secondary reaction from singlet oxygen to triplet oxygen causes the red glow. It is a fast reaction. At atmospheric pressure, pure singlet oxygen gas is converted to triplet oxygen in microseconds, due to the many molecular collisions.
Slower release of hypochlorite, moderation of reaction
When calcium hypochlorite is used, then a very violent reaction occurs, with a fairly bright but also short-lasting burst of red light. Better results are obtained, when the hypochlorite is not used up at once, but some form of moderation could be used. For this purpose, the swimming pool chemicals sodium dichloroisocyanurate (Na-DCCA) en trichloroisocyanuric acid (TCCA) come in handy.
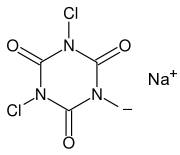
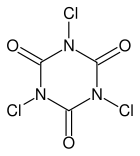
This type of molecules react with water in an equilibrium reaction, where the chlorine atoms can be replaced by a hydrogen atom, while the water molecule is converted to hypochlorous acid.
۞-Cl + H2O ↔ ۞-H + HOCl
Here ۞ stands for the ring-shaped radical without the chlorine atom. Such a reaction is possible at both chlorine atoms of Na-DCCA and at all three chlorine atoms of TCCA. In this way, hypochlorite becomes available again, in the form of HOCl and this then in turn reacts with hydrogen peroxide to give singlet oxygen.
With TCCA, this reaction is quite slow, because of the low solubility of TCCA in water. This makes the red glow of the reaction with TCCA somewhat weak. With Na-DCCA the reaction speed is ideal. The production of HOCl is at a sufficiently fast rate to obtain a real good red glow, while at the same time, it is not so fast, that the reaction becomes too violent. For this reason, Na-DCCA gives best results.