


Chemical chameleon
When a very dilute solution of permanganate is slowly reduced to colloidally dispersed manganese (IV) oxide, then a beautiful range of colors is traversed, starting from purple/violet and ending in yellow/brown. It is amazing to see the solution change color. For this reason, in the past, permanganate sometimes was called 'chemical chameleon'. Only at a later time, it was understood that this remarkable phenomenon just is the result of change of oxidation state, and the mixing of colors of different compounds. From a chemical point of view, this experiment is not that special, but it still makes a nice display, maybe even more so, now it is well understood.
![]()
![]() Required
chemicals:
Required
chemicals:
-
potassium permanganate
-
saccharose (plain sugar, also called sucrose)
-
sodium hydroxide
![]() Required
equipment:
Required
equipment:
-
glass beaker or erlenmeyer.
-
test tube
![]() Safety:
Safety:
- Potassium permanganate is a strong oxidizer. However, in this experiment a very dilute solution is used (less than 0.05%), so there is no real risk.
- Sodium hydroxide is very corrosive to human tissue. Be careful with the solid and concentrated solutions. If any of this solids comes in contact with the skin, then rinse with water, until the slippery feeling is gone.
![]() Disposal
and cleanup:
Disposal
and cleanup:
- The waste of this experiment only contains mg quantities of manganese and as such it can be flushed down the drain.
- If any brown stains of manganese dioxide remain on the glassware after the experiment, then rinse with a dilute hydrochloric acid (5% or so), to which a small amount of sodium sulfite or hydrogen peroxide is added. This liquid removes brown stains of manganese dioxide at once.
![]()
Preparation for the experiment
![]() Dissolve
a very small amount of potassium permanganate in a few ml of water in a test
tube. Only use a few mm³ of solid. It really is important not to use too much
potassium permanganate, otherwise the solutions simply looks black and then the
experiment is not really impressive. The colors will be too dark in that case.
Dissolve
a very small amount of potassium permanganate in a few ml of water in a test
tube. Only use a few mm³ of solid. It really is important not to use too much
potassium permanganate, otherwise the solutions simply looks black and then the
experiment is not really impressive. The colors will be too dark in that case.
![]() Put 100 ml of water in the erlenmeyer and add a spatula full
of solid sodium hydroxide (500 mg or so) and add three spatula's full of sugar.
Dissolve all the solid material, such that a colorless and clear solution is
obtained.
Put 100 ml of water in the erlenmeyer and add a spatula full
of solid sodium hydroxide (500 mg or so) and add three spatula's full of sugar.
Dissolve all the solid material, such that a colorless and clear solution is
obtained.
![]()
Change of color
Pour the contents of the test tube into the 100 ml of sugar/NaOH solution and swirl, such that the liquid is mixed well. Then let the beaker stand for a while and watch the color change from purple to yellow/brown. This takes a few minutes, the exact period depending on temperature and concentration of the reactants.
Below follow the pictures of all changes. Between the pictures, there is an interval of time of approximately 10 seconds, but precise times cannot be predicted easily. In another run, the change of colors may go faster or slower, but the order and type of colors always will be the same.












When put side by side as small pictures, a nice band of colors is obtained:












The color first darkens, then it goes to green and gradually it shifts towards yellow/brown.
![]()
Discussion of results
Permanganate is slowly reduced by sugar in alkaline environments. Sugar is an organic compound, having many -OH groups, attached to carbon atoms, which also have a hydrogen atom attached directly to it. Such organic compounds, containing C(H)(OH) structures (secondary alcohol groups) are easily oxidized.
The structure of sugar is shown here:
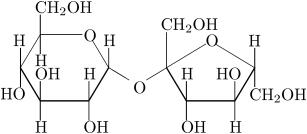
The oxidation of the C(H)(OH) structure is as follows, where the alcohol-group is oxidized to a ketone-group:
C(H)(OH) + 2OH → C(=O) + 2H2O + 2e
This reaction requires hydroxide ions. The observed speed of the reaction indeed is strongly depending on the concentration of sodium hydroxide. When a lot of sodium hydroxide is dissolved, e.g. a teaspoon full of solid, then the first part of the reaction only takes a few seconds instead of tens of seconds.
In alkaline environments, permanganate ion first is reduced to manganate ion:
MnO4 + e → MnO42
The left is deep purple, the right is deep green. When both are present, then light in the red end of the spectrum is absorbed by the green manganate, and at the same time, light at the blue end of the spectrum is absorbed by the violet permanganate. This combination of absorptions make the solution almost appear black, hence the darkening at the start of the experiment. When almost all permanganate is reduced to manganate, then the liquid looks beautifully deep green.
When there is excess sugar, then the manganate in turn is reduced further as follows:
MnO42 + 2H2O + 2e → MnO2 + 4OH
At the very low concentrations, used in this experiment, the MnO2 does not precipitate, but a colloidal solution of hydrous MnO2 is formed, which remains clear. Hydrous MnO2 is brown, but at the low concentrations, used in this experiment, it is more yellow than brown.