


Beauty of chemistry
This web page describes a few simple
experiments, brought in a special way. The experiments
are the following:
- Dissolving copper metal in concentrated nitric acid. This reaction is filmed at high speed (1000 fps) and when this is done, then many interesting details are revealed. The final product of the reaction also allows making beautiful pictures with remarkable color contrasts.
- Dissolve iodine pentoxide in a concentrated solution of hydrazine dihydrochloride. This extremely violent and exothermic reaction also is filmed at 1000 fps.
- Use the exothermic reaction between
chlorine gas and red phosphorus to set off a flash
mix. The combination of different reactions into one
experiment makes this a particularly impressive
demonstration, which can easily be done and can be
carried out safely.
Nitric acid cannot be obtained anymore
in the EU, but it can fairly easily be distilled from
sodium nitrate or potassium nitrate and sulfuric acid in
an all glass distillation apparatus. Another option is
to mix sodium bisulfate monohydrate (common pH-minus for
swimming pools) and sodium nitrate and distill off
nitric acid from this mix. Only a small quantity is
needed. I distilled a little yellow fuming nitric acid
(appr. 30 ml) and diluted this for the experiment to
appr. 60% by weight.
![]()
![]() Required chemicals:
Required chemicals:
- copper metal (e.g. from flexible
electrical wire, from which the plastic isolation is
removed)
- nitric acid (to be prepared from
sodium nitrate and sulfuric acid), HNO3
- iodine pentoxide, I2O5
- hydrazine dihydrochloride, N2H4·2HCl
- calcium hypochlorite (swimming pool
chlorinator), Ca(OCl)2
- dilute hydrochloric acid
- aluminium powder
- red phosphorus
- potassium perchlorate (can be
substituted by potassium nitrate)
![]() Required equipment:
Required equipment:
- erlenmeyer
- test tubes
![]() Safety:
Safety:
- All of these experiments produce fumes or gases, which should not be inhaled. Quantities are small, so there is not a really high risk, but the experiments should be carried out at least in a well-ventilated room. Even better is the use of a fume hood.
- Nitric acid is corrosive. Distilling the acid also must be done with care. Be absolutely sure not to get any of the hot acid on your skin or eyes. Wear appropriate protection.
- Iodine pentoxide is corrosive and
also is a strong oxidizer, which gives nasty stains on
skin (it is reduced to iodine by grease and cells from
the skin).
- Hydrazine and its compounds are suspected carcinogens. Avoid exposure. In this experiment, however, the hydrazine will not make it into the air. Excess iodine is expelled and this assures total destruction of any hydrazine, which might be released into the air.
- Red phosphorus is very flammable.
![]() Disposal:
Disposal:
- The waste of the dissolved copper
should be neutralized with a little sodium
bicarbonate, until fizzling stops and then be treated
as heavy metal waste. The waste of the other
experiments can be flushed down the drain with a lot
of water. Any iodine-waste from the second experiment
can be neutralized easily with some sodium sulfite
before it is flushed away.
![]()
Bubbling of copper metal
in concentrated (not fuming) nitric acid
This is a simple
experiment. A piece of copper is added to concentrated
nitric acid and the reaction is watched. It is a very
well-known experiment, which also is demonstrated in
many high schools, but this classic remains very
interesting and when viewed at 40x slow motion, a whole
new world opens up on how the reaction starts and how
the bubbles move through the liquid.
![]() Pour a few ml of concentrated
nitric acid (appr. 60% by weight) in a test tube.
Home-made nitric acid (distilled from sodium nitrate),
usually is more concentrated. This must be diluted with
some water for a smooth and fast reaction.
Pour a few ml of concentrated
nitric acid (appr. 60% by weight) in a test tube.
Home-made nitric acid (distilled from sodium nitrate),
usually is more concentrated. This must be diluted with
some water for a smooth and fast reaction.
![]() Heat the nitric acid somewhat.
It should not be boiling hot, a temperature of 60 °C or
so is fine. No need to be precise with this, a good
guideline is having the test tube just a little too hot
to touch it for longer than a second.
Heat the nitric acid somewhat.
It should not be boiling hot, a temperature of 60 °C or
so is fine. No need to be precise with this, a good
guideline is having the test tube just a little too hot
to touch it for longer than a second.
![]() Make the copper wire into a
small ball and then let it fall into the acid.
Make the copper wire into a
small ball and then let it fall into the acid.
As soon as the copper falls
into the acid, a violent reaction sets in. A video at
real speed can be downloaded here.
The video at 40x slow
motion is much more impressive. Below follow three
images of the formation of the bubbles, immediately
after adding the copper to the acid.



In a few tens of seconds all of the copper is dissolved
and a bright blue solution is obtained.


The final color contrast is really striking:

![]()
Reaction between iodine
pentoxide and hydrazine
The reagents of this experiment are
less common, but the experiment itself is simple again.
A piece of solid iodine pentoxide is dropped into a
concentrated solution of hydrazine dihydrochloride. This
results in an extremely violent and exothermic reaction
in which nitrogen and iodine are formed. So much heat is
produced, that a large part of the iodine escapes as a
plume of purple gas.
![]() Prepare a few ml of a nearly
saturated solution of hydrazine dihydrochloride. The
liquid cools down a little bit when the solid is
dissolved. There is no need to heat the liquid in this
experiment.
Prepare a few ml of a nearly
saturated solution of hydrazine dihydrochloride. The
liquid cools down a little bit when the solid is
dissolved. There is no need to heat the liquid in this
experiment.
![]() Drop a piece of solid iodine
pentoxide in the cold solution of hydrazine
dihydrochloride.
Drop a piece of solid iodine
pentoxide in the cold solution of hydrazine
dihydrochloride.
As soon as the piece of
iodine pentoxide falls in the solution, the reaction
starts. Immediately, a big bubble of nitrogen is formed
and even in this short time from the start of the
reaction (just a few tens of ms), a clearly visible
purple color of iodine vapor can be observed. This
picture was made with a high speed camera at 1000 fps.

The reaction itself is quite
interesting to watch, even without high speed camera. A
big plume of purple iodine vapor escapes from the test
tube:
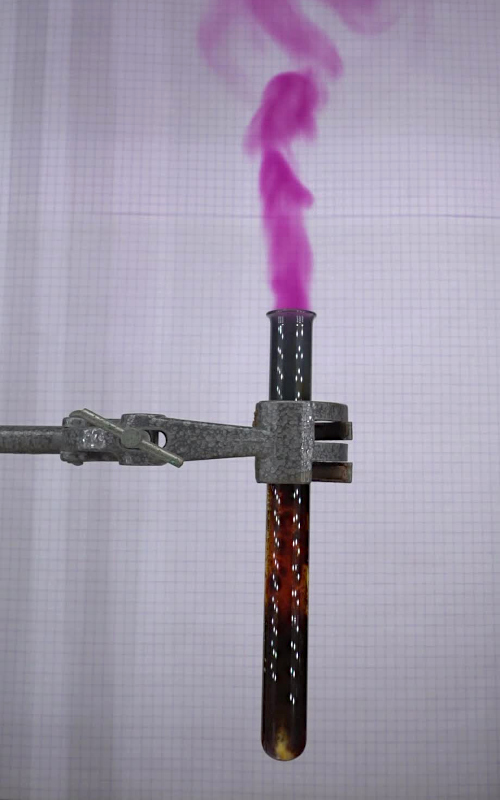
A video of this reaction is
available in normal time, showing the plume of iodine,
and a close-up video of
this reaction at 40x slow motion shows the
formation of bubbles and iodine in the test tube.
![]()
Ignition of flash
powder by chlorine gas
This experiment combines several
reactions into an impressive experiment, which also
works very well as a demonstration experiment. It is
well-known that red phosphorus reacts exothermically
with halogens. With chlorine the reaction is so
exothermic, that the red phosphorus ignites. A mix of
aluminium powder and potassium perchlorate (a flash mix)
burns extremely fast and hot, with a white flash. The
flash mix, however, is somewhat hard to ignite. The heat
of burning red phosphorus, however, is sufficient to
ignite the flash mix, if the phosphorus is sufficiently
finely divided throughout the mix. So, for this
experiment, a tiny amount of red phosphorus is mixed
with aluminium and finely powdered potassium
perchlorate. Simply immersing this mix in chlorine gas
sets off the mix.
Note 1: A mix of red phosphorus and potassium perchlorate can be handled fairly safely, as long as the potassium perchlorate does not contain any chlorate impurity. For this experiment only a few tens of mg are mixed, so the risk is small anyway.
Note 2: In the EU, potassium
perchlorate and sodium perchlorate cannot be obtained
anymore. Potassium nitrate can be used instead, but the
reaction is somewhat less spectacular. The flashes with
the nitrate are less intense. For this experiment, I
prepared a little potassium perchlorate from waste of
other experiments. Perchloric acid can be obtained
legally in the EU and I used that for other experiments
on transition metal complexes and the waste of these
experiments I kept and from that I made a small quantity
of potassium perchlorate (this is the only perchlorate
which can easily be made from that waste in a pure
state, due to its low solubility in cold water).
Ammonium perchlorate also can be obtained legally and
that can also be used to make small quantities of
potassium perchlorate. Using ammonium perchlorate
instead of potassium perchlorate does not give good
results. The mix with ammonium perchlorate burns much
slower. If you really need to use a substitute, then use
potassium nitrate. Do not use chlorate!
![]() Prepare a little amount of
flash mix, with some phosphorus mixed in as well. In
order to do so, mix a spatula full of finely powdered
potassium perchlorate with half a spatula full of
aluminium powder and a tiny amount (10% to 20% of the
amount of aluminium) of finely powdered dry red
phosphorus. Carefully swirl the mix in a petri dish or
an aluminium holder for a tea light. Do not grind!
The precise ratio of chemicals is not critical.
Prepare a little amount of
flash mix, with some phosphorus mixed in as well. In
order to do so, mix a spatula full of finely powdered
potassium perchlorate with half a spatula full of
aluminium powder and a tiny amount (10% to 20% of the
amount of aluminium) of finely powdered dry red
phosphorus. Carefully swirl the mix in a petri dish or
an aluminium holder for a tea light. Do not grind!
The precise ratio of chemicals is not critical.
![]() Pour 20 ml or so of dilute
hydrochloric acid in an erlenmeyer.
Pour 20 ml or so of dilute
hydrochloric acid in an erlenmeyer.
![]() Add a teaspoon of granular
calcium hypochlorite to the acid. Adding 10 ml of
concentrated bleach also works. Use of TCCA is not
recommended, because of foaming. Wait till the
erlenmeyer is full of chlorine gas. Avoid inhaling the
gas!
Add a teaspoon of granular
calcium hypochlorite to the acid. Adding 10 ml of
concentrated bleach also works. Use of TCCA is not
recommended, because of foaming. Wait till the
erlenmeyer is full of chlorine gas. Avoid inhaling the
gas!
![]() Using a metal spatula, pour
some of the powdered flash mix with phosphorus into the
chlorine gas in the erlenmeyer.
Using a metal spatula, pour
some of the powdered flash mix with phosphorus into the
chlorine gas in the erlenmeyer.
When the finely powdered mix is immersed in the chlorine
gas, then the exothermic reaction between the chlorine
and the red phosphorus in the mix sets off the flash mix
and this gives a really spectacular effect. Sometimes
you even get mushroom clouds, like in a nuclear
explosion. The following pictures were taken with
intervals of 10 ms between two of them.





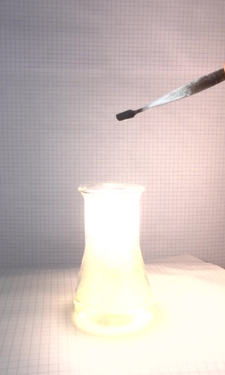

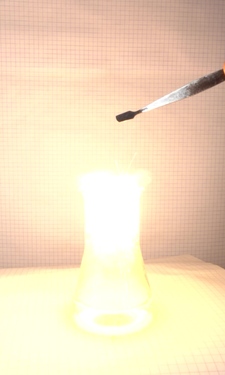



















Another set of pictures was made, also with 10 ms intervals between images. In that series, there are many sparks, and the mix used in that experiment burns even hotter.









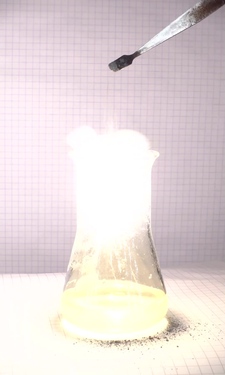


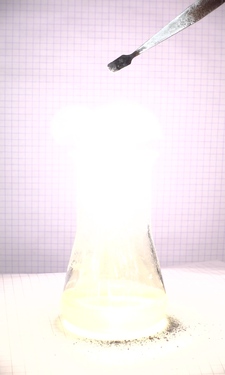











Two videos can be downloaded of this reaction: video1 and video2.
![]()
Discussion of results
![]() In
the first experiment, copper reacts with nitric acid,
giving copper(II) ions in solution and nitrogen dioxide
gas. Under the conditions in this experiment (quite warm
and also quite concentrated acid), the main gaseous
product, produced in the reaction between copper metal
and nitric acid is the brown NO2 gas. The
reaction equation is:
In
the first experiment, copper reacts with nitric acid,
giving copper(II) ions in solution and nitrogen dioxide
gas. Under the conditions in this experiment (quite warm
and also quite concentrated acid), the main gaseous
product, produced in the reaction between copper metal
and nitric acid is the brown NO2 gas. The
reaction equation is:
Cu + 4 HNO3 → Cu2+ +
2 NO3–
+ 2 NO2
+ 2 H2O
3 Cu
+ 8 HNO3 → 3 Cu2+
+ 6 NO3–
+ 2 NO + 4 H2O
In practice, none
of the above reactions occurs exclusively, there will
always be a combination of both reactions, at the
start, when the acid is still concentrated, mainly the
first one and at the end mainly the second one. So,
this reaction between copper metal and nitric acid is
not really suitable to make pure NO2 or
pure NO, one gets a mix of these gases.
![]() In the second experiment, iodine pentoxide
reacts with hydrazine. In solid hydrazine
dihydrochloride, the hydrazine is protonated twice,
present as an ion +H3N-NH3+.
In solution, this ion decomposes and when the solid salt
is dissolved in water, then the compound ionizes as
follows:
In the second experiment, iodine pentoxide
reacts with hydrazine. In solid hydrazine
dihydrochloride, the hydrazine is protonated twice,
present as an ion +H3N-NH3+.
In solution, this ion decomposes and when the solid salt
is dissolved in water, then the compound ionizes as
follows:
N2H4·2HCl → N2H5+ + H+ + 2 Cl–
The solution becomes strongly
acidic. It contains nearly no free hydrazine. Hydrazine
is a strong reductor, regardless of whether it is
protonated or not. Iodine pentoxide, on the other hand,
is a strong and very facile oxidizer, and hence, when
these two compounds are brought in contact with each
other a very violent and exothermic reaction occurs:
3 N2H5+ + I2O5 →
3 N2 + 2 I–
+ 5 H+
+ 5 H2O + heat
When there is excess iodine
pentoxide, then any iodide reacts with iodine pentoxide
to iodine in the presence of acid. This explains the
formation of all the iodine vapor. On the solid piece of
iodine pentoxide, which also is covered with a lot of
gaseous nitrogen, the amount of hydrazine quickly is
used up and any iodide ion then is quickly oxidized to
iodine:
10 I–
+ I2O5 + 10 H+
→ 6 I2
+ 5 H2O
Due to the heat, produced in
the reaction with hydrazine, the iodine partially
evaporates and escapes as purple gas. The rest remains
in solution as brown iodine and some is precipitated as
nearly black solid.
![]() In the third experiment,
phosphorus reacts with chlorine, producing heat, and
this in turn ingites the flash mix of aluminium and
potassium perchlorate:
In the third experiment,
phosphorus reacts with chlorine, producing heat, and
this in turn ingites the flash mix of aluminium and
potassium perchlorate:
2 P + 3 Cl2 →
2 PCl3 + a lot of heat
The heat is sufficient to ignite the
flash mix:
8 Al + 3 KClO4 → 4 Al2O3 + 3 KCl + much more heat
The reaction between aluminium and
potassium perchlorate produces a really large amount
of energy, leading to white hot particles and
emission of a lot of light. The loose powder,
falling freely, does not really give an explosion,
but a finely powdered intimate mix of aluminium and
potassium perchlorate, when in a pile on a spoon,
can explode, due to self-confining, once it is
ignited.
The phosphorus in the mix makes it somewhat less
energetic. In the first set of images, one can see
that the light has a somewhat yellowish cast. This
is because a little too much phosphorus was added to
the mix. In the second set of images, one can
clearly see that a somewhat more carefully prepared
flash mix has even brighter light.