


Barking dog: N2O and CS2
This is a classical experiment, but also a very spectacular experiment. This is a nice demonstration that there are other gases besides oxygen which support combustion.

The picture above shows a graduated cylinder of 100 ml, filled with a mix of nitrous oxide and carbon disulfide vapor and it shows the extremely bright light, produced from the burning mix of this gas and vapor.

 This
is a beautiful experiment, but it is a fairly dangerous experiment as well.
Carbon disulfide is poisonous, one must be careful not to inhale too much of the
vapor. Carbon disulfide also is extremely flammable. Any open source of heat is
a serious risk, even boiling hot water may be capable of igniting carbon
disulfide. So, be sure that no strong heat sources are present, when the carbon
disulfide is handled. Best is to use a very well-ventilated room or a fume hood.
This
is a beautiful experiment, but it is a fairly dangerous experiment as well.
Carbon disulfide is poisonous, one must be careful not to inhale too much of the
vapor. Carbon disulfide also is extremely flammable. Any open source of heat is
a serious risk, even boiling hot water may be capable of igniting carbon
disulfide. So, be sure that no strong heat sources are present, when the carbon
disulfide is handled. Best is to use a very well-ventilated room or a fume hood.
![]()
![]() Required
chemicals:
Required
chemicals:
-
nitrous oxide (whipped cream chargers are suitable for this)
-
carbon disulfide
![]() Required
equipment:
Required
equipment:
-
long glass tube and suitable rubber stopper(s)
-
long/tall beaker or graduated cylinder
![]() Safety:
Safety:
-
carbon disulfide is poisonous, avoid inhaling of the vapor;
-
carbon disulfide is extremely flammable;
-
sulphur dioxide is released, this has a strong pungent smell and in higher concentrations this is toxic.
![]() Disposal:
Disposal:
- There is no waste, which needs to be disposed of in a special way. The glass tubing becomes covered with a thin sulphur layer. This can be scrubbed off easily and any waste from this can be disposed of as ordinary household waste.
![]()
The classical barking dog experiment
The most spectacular experiment is the use of a long glass tube, filled with nitrous oxide to which some carbon disulfide is added, and which then is ignited.
![]() Take a
long glass tube (3 cm diameter, 50 cm length and thick-walled) and stopper this
on one side. Fill this with nitrous oxide from a whipped cream dispenser. The
setup as shown in the picture below is suitable. The whipped cream dispenser is
filled with nitrous oxide from a whipped cream charger. The gas is available under
high pressure in the container. It can be released slowly by pressing the handle
and it is blown in the glass tube through a thin plastic tube.
Take a
long glass tube (3 cm diameter, 50 cm length and thick-walled) and stopper this
on one side. Fill this with nitrous oxide from a whipped cream dispenser. The
setup as shown in the picture below is suitable. The whipped cream dispenser is
filled with nitrous oxide from a whipped cream charger. The gas is available under
high pressure in the container. It can be released slowly by pressing the handle
and it is blown in the glass tube through a thin plastic tube.

Filling is demonstrated by this video (download size is appr. 1.6 MByte).
![]() When the
glass tube is filled with nitrous oxide, then add 1 ml of carbon disulfide and
swirl the tube, while it is stoppered on both sides.
When the
glass tube is filled with nitrous oxide, then add 1 ml of carbon disulfide and
swirl the tube, while it is stoppered on both sides.
![]() Remove
one of the stoppers from the tube, and ignite the vapor/gas mix. This results in
burning of the gas/vapor mix with an intensely bright blue light and a
roaring/barking noise from the tube. The 12 pictures below show a sequence of
frames, from the moment of ignition of the gas/vapor mix, until all has burnt. Total time is only 400
ms.
Remove
one of the stoppers from the tube, and ignite the vapor/gas mix. This results in
burning of the gas/vapor mix with an intensely bright blue light and a
roaring/barking noise from the tube. The 12 pictures below show a sequence of
frames, from the moment of ignition of the gas/vapor mix, until all has burnt. Total time is only 400
ms.





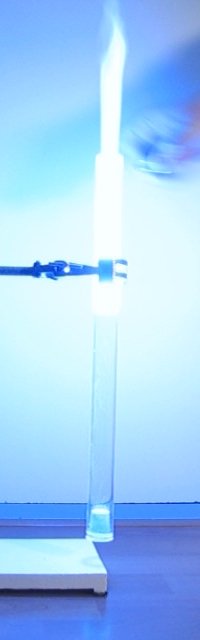
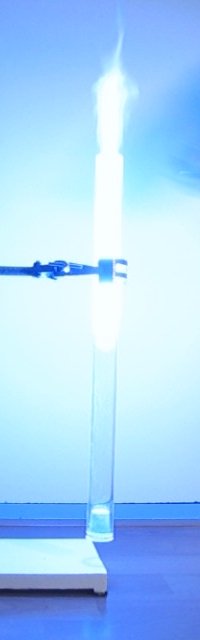
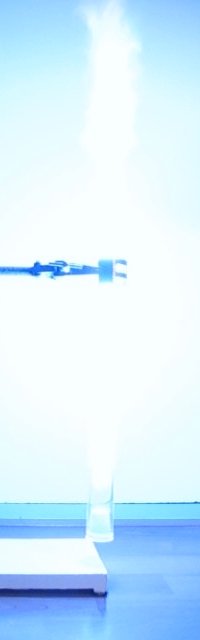




A video of this experiment demonstrates the barking dog sound produced during burning. Download size of the video is 531 kByte. The light is impressive, but the sound is equally impressive, it really adds to the fun of the demonstration.
After the burning, the tube is covered by a yellow layer of sulphur. Immediately after burning it is fairly bright/dark yellow, but on cooling down, the layer becomes lighter, as shown by the picture below.

![]()
A small scale version of this experiment
The barking dog experiment is most spectacular, but also somewhat dangerous. This experiment can also be performed on a small scale. The effect already is quite spectacular, even on a 100 ml scale.
For this small scale version of the experiment, a cylinder with a volume of 100 to 150 ml must be filled with nitrous oxide and 0.5 ml of carbon disulfide must be added. The cylinder then must be swirled in order to mix the gas and vapor sufficiently well, and then the gas/vapor mix can be ignited. The picture below shows a graduated cylinder, filled with a gas/vapor mix. Covering the cylinder with a petri dish is suitable, when it is swirled around, as long as the petri dish covers the cylinder sufficiently well.

The four pictures below show the end of the result of igniting the gas/vapor mix in the cylinder. These pictures only show the tail of the reaction. During the main part of the reaction so much light was emitted, that the pictures were rather overexposed.




After the reaction the cylinder is covered with a yellow layer of sulphur. A video of this reaction also demonstrates the whoosh sound, made with this small graduated cylinder. There is no such strong barking dog sound, but still there is a clearly audible noise when the gas/vapor mix is ignited. Download size of the video is 660 kByte.
After the reaction and after cooling down, the graduated cylinder looks as follows:

Again, after cooling down, the color of the sulphur, which covers the inside of the glass, becomes lighter.
![]()
Discussion of results
Nitrous oxide supports combustion of many organic compounds, and when the gas is pure, then it supports combustion even better than plain air.
Carbon disulfide also burns very well in this gas. There is not a single nice clean reaction. Carbon disulfide can burn incompletely in nitrous oxide, resulting in formation of elemental sulphur
![]() In case of excess N2O there is complete
combustion:
In case of excess N2O there is complete
combustion:
CS2 + 6N2O → CO2 + 2SO2 + 6N2
![]() When
there is excess CS2:
When
there is excess CS2:
CS2 + (6 - 2n)N2O → CO2 + (2-n)SO2 + nS + (6-2n)N2
The value of n depends on the strength of the excess amount (0 ≤ n ≤ 2). In the experiments, described on this page, there was quite some excess carbon disulfide and hence the formation of a clearly visible layer of sulphur, and also some off-white smoke is produced (not visible in the pictures of videos).
No carbon soot is produced. Apparently, in carbon disulfide, the carbon first is converted to carbon dioxide and then the sulphur is converted. When the gas mix is very low in nitrous oxide, then even some carbon monoxide may be formed, but never some soot is produced.