


Arsenic sulfides: As4S4 and As2S3
On this web page some chemical
properties of the two most common arsenic sulfides are
demonstrated. In the past, both sulfides were used as
pigments. In many Renaissance paintings, these pigments
can be found for yellow and orange colors. Nowadays,
these compounds hardly are used anymore, due to their
great toxicity, and also due to their limited stability
towards other chemicals and light. There are better
alternatives now for yellow and orange colors.
In the bulk state, As4S4,
called realgar, is a bright red crystalline compound,
often covered in a thin layer of orange powder. Realgar
crystals slowly fall apart into fine powder on exposure
to bright light of wavelengths in the range of 500 nm to
670 nm. This powder is ochre/orange, and it is an
allotrope of realgar, called pararealgar.
Realgar is known already for a very
long time. Even in biblical times it was known already,
named σανδαράκη (sandarákē,
“realgar”). It was mixed with wax to make seals
for envelopes of important documents and it was a
highly valued mineral. It was also used as a
poison.
The other sulfide, As2S3,
is called orpiment. It also is known for a very long
time already. In the Roman empire it was called auripigmentum. From this, the word
orpiment is formed over the centuries. This compound was
also used for making seals for important documents, and
it also was used to make the tips of arrows toxic. In
ancient times it was already known that orpiment is
toxic.
The following picture shows crystals
of both realgar and orpiment. The grid on the paper has a side of 5
mm.The crystals both have a natural origin. It is
remarkable that a natural compound can be so pure.

In the experiments, described on this web page, crunched
crystals of these minerals are used. Both crystalline
solids are very brittle and quite soft. With a metal
spoon and some pressure, it is easy to crack crystals
like these. On AliExpress, I could obtain 100 grams of
crunched orpiment crystals for less than $10, including
shipping. Small crystals of chinese orpiment are quite
cheap. Realgar is much more expensive and large crystals
of orpiment, like the one shown in the picture also are
more expensive. The cheap orpiment from China, however,
has very good purity, especially if one considers the
fact that it is a natural compound. Below, a sample of
100 grams of small crystals of orpiment is shown. This
material is used in the experiments, described further
below.

I also crushed a small piece of realgar with a metal
spoon and after that, I used a plastic tool to make it
finer. The color changes from red to orange, when the
particles become finer. Lateron in the experiments, it
becomes clear, however, that the crunching of the
realgar was not perfect, still some larger particles
remained, which were hard to dissolve.
 The
experiments on this webpage are interesting and show
some colorful chemistry of the element arsenic.
Experiments with arsenic are not common at all on the
internet and most amateur chemists do not have any
arsenic compounds. The experiments, however, must be
carried out with great care. Avoid exposure to powdered
realgar or orpiment and be sure not to get any of the
solutions in this experiment on your skin. If you get an
arsenical solution on your skin, quickly rinse it with
water for several minutes.
The
experiments on this webpage are interesting and show
some colorful chemistry of the element arsenic.
Experiments with arsenic are not common at all on the
internet and most amateur chemists do not have any
arsenic compounds. The experiments, however, must be
carried out with great care. Avoid exposure to powdered
realgar or orpiment and be sure not to get any of the
solutions in this experiment on your skin. If you get an
arsenical solution on your skin, quickly rinse it with
water for several minutes.
With the discovery that orpiment is
quite easy to obtain for decent prices, the element
arsenic has become much more accessible than it was
otherwise. The element and its compounds afford some
interesting experiments, but should only be handled by
the somewhat more experienced home chemist.
![]()
![]() Required chemicals:
Required chemicals:
- arsenic sulfide, As4S4, realgar
- arsenic sulfide, As2S3, orpiment
- sodium hydroxide
- bleach
- hydrochloric acid
![]() Required equipment:
Required equipment:
- test tubes
- beakers
- magnetic stirrer
![]() Safety
Safety
- Both arsenic sulfides are toxic
- The solutions are particularly toxic, especially the alkaline ones, because they attack skin and arsenic can be absorbed easily through them. In case of contact with these solutions, rinse with water for a long time, until the slippery feeling is gone.
- Avoid inhalation of powdered arsenic sulfides, when crushing the crystals.
- Sodium hydroxide is very corrosive to skin and especially to the eyes. Avoid contact with the eyes at any cost. If sodium hydroxide comes in contact with the skin, rinse with water, until the slippery feeling disappears.
- Hydrochloric acid is corrosive.
 Do not add strong reductors like zinc
or sodium borohydride to the solutions, containing
arsenic! The use of such reductors usually leads to
formation of hydrogen, and when arsenic is present in
solution, then small amounts of arsine, AsH3,
may be formed, which is extremely poisonous!
Do not add strong reductors like zinc
or sodium borohydride to the solutions, containing
arsenic! The use of such reductors usually leads to
formation of hydrogen, and when arsenic is present in
solution, then small amounts of arsine, AsH3,
may be formed, which is extremely poisonous!
![]() Disposal:
Disposal:
- The waste should be collected and
not be flushed down the drain. Bring the collected
waste to a proper waste processing facility.
![]()
Dissolving realgar
For this experiment, powdered realgar
(crunched crystals) are used. A closeup is shown here:

Realgar is soluble in solutions of sodium hydroxide. The
solutions should be warm. The reaction is not very fast,
but finally, all realgar can be dissolved. Initially,
the solid dissolves with a very deep red color, but very
quickly (in boiling hot solutions of NaOH this only
takes a few seconds), the solution decomposes and a dark
brown precipitate is formed.
![]() Prepare a solution of
approximately 10% sodium hydroxide by weight.
Prepare a solution of
approximately 10% sodium hydroxide by weight.
![]() Heat this liquid, so that it
becomes quite hot, but not so hot that it is close to
boiling.
Heat this liquid, so that it
becomes quite hot, but not so hot that it is close to
boiling.
![]() To this liquid add a little
crushed realgar.
To this liquid add a little
crushed realgar.
The realgar slowly dissolves,
giving first a chololate brown color, but when more
dissolves, it becomes nearly black. Below follow a few
videos, of the dissolving of the realgar.
- Adding realgar to warm 10% solution of sodium hydroxide
- Approximately 1.5 minute later
- Approximately 3 minutes later
This experiment also shows that
realgar is quite hydrophobic. It is not easily wetted
and remains floating on the surface of the solution.
This also hinders quite dissolving of the material.
![]() Another experiment
was done by adding a boiling hot and more concentrated
solution (appr. 20% by weight) of sodium hydroxide to
some solid realgar in a test tube. This is shown in a video,
where one sees a transient deep red color, but very
quickly the solution becomes very dark brown and
turbid.
Another experiment
was done by adding a boiling hot and more concentrated
solution (appr. 20% by weight) of sodium hydroxide to
some solid realgar in a test tube. This is shown in a video,
where one sees a transient deep red color, but very
quickly the solution becomes very dark brown and
turbid.
The contents of the test tube and the contents of the beaker were added to each other and put in a test tube, which was swirled to make an homogenous liquid with the dark brown precipitate. This test tube was allowed to stand for two days. After two days, a dark brown precipitate is in the bottom of the test tube, together with a smaller quantity, sticking to the glass (the test tube was not positioned perfectly vertically). The precipitate is very fine.

Dissolving elemental
arsenic
The brown solid, formed in these
solutions, is elemental arsenic, very finely divided. On
standing, it forms a flocculent precipitate. This
precipitate can easily be dissolved in bleach. This can
easily be demonstrated.
![]() Swirl
the test tube with the very dark brown precipitate
from the previous experiment. Take approximately 25%
of the dark liquid from the test tube and pour this in
a small beaker.
Swirl
the test tube with the very dark brown precipitate
from the previous experiment. Take approximately 25%
of the dark liquid from the test tube and pour this in
a small beaker.
![]() To
this turbid brown liquid add some bleach (with
approximately 10% active chlorine), while
stirring.
To
this turbid brown liquid add some bleach (with
approximately 10% active chlorine), while
stirring.
The stirring solution quickly turns
much paler. the arsenic dissolves, but some milky
turbidity is formed. The solution also contains
sulfide, and this is oxidized. Some of this
sulfide is oxidized to sulfur, while most is
oxidized further to (most likely) sulfate. The
sulfur becomes visible as pale turbidity. The
below images show the color of the solution with
intervals of one to two seconds.






In a video of the dissolving arsenic you can see this change of color over a period of 25 seconds. The video also shows that the crushed realgar was not crushed perfectly. Some larger red crystalline pieces can be seen on the bottom of the small beaker. The video also shows that the coarser pieces of realgar do not readily dissolve in the bleach.
![]()
Dissolving orpiment
Orpiment is more easily
dissolved in hot dilute solutions of sodium hydroxide
than realgar. Even somewhat bigger crystals of orpiment
can be dissolved in boiling hot solutions of sodium
hydroxide in a minute or so.
![]() Put some orpiment in a test
tube.
Put some orpiment in a test
tube.
![]() Add some water.
Add some water.

The picture shows that the
larger pieces of orpiment collect at the bottom of the
test tube, but the finer particles remain floating on
the water. Just like realgar, the orpiment also is not
that easily wetted.
![]() Add approximately 20% by
weight of sodium hydroxide. This is not critical at all,
it certainly will also work with 10% or 30%.
Add approximately 20% by
weight of sodium hydroxide. This is not critical at all,
it certainly will also work with 10% or 30%.
![]() Carefully heat the test tube
with the sodium hydroxide, water, and orpiment. Swirl
well, while heating. Stopper the test tube loosely with
a piece of wadding to avoid formation of small droplets
with arsenic before heating. If the test liquid boils,
then the small droplets do not escape into the air,
while any water vapor and hot air can escape from the
test tube.
Carefully heat the test tube
with the sodium hydroxide, water, and orpiment. Swirl
well, while heating. Stopper the test tube loosely with
a piece of wadding to avoid formation of small droplets
with arsenic before heating. If the test liquid boils,
then the small droplets do not escape into the air,
while any water vapor and hot air can escape from the
test tube.
After approximately 1 minute
of heating and swirling all of the orpiment has
dissolved, including the larger pieces at the bottom.
The resulting liquid is nearly clear, it contains a very
small amount of brown solid, which settles at the bottom
after a while. The liquid itself is very pale yellow.

The amount of brown material
is really small. This most likely is due to tiny amounts
of realgar, as an impurity of the orpiment. Realgar
impurity is quite common with natural orpiment as shown
below.

Formation
of arsenic sulfide in acidic solution
Arsenic sulfide actually is quite a remarkable compound. Many sulfides have the property that they are stable at high pH, but are decomposed in acids. With orpiment, arsenic(III) sulfide, this is the other way around. As the above experiment shows, orpiment can easily be dissolved in alkaline solutions. When such a solution is added to an acid, then arsenic sulfide (of higher purity and purer color) precipitates again. This experiment is really remarkable, because the binding of the sulfide is so strong, that there only is a very faint smell of hydrogen sulfide on addition of the sulfide solution to dilute acid. The experiment is as follows:
![]() Prepare a solution of 5%
hydrochloric acid (actually, any strong acid will do).
Prepare 50 ml or so of the acidic solution and put
this in a beaker.
Prepare a solution of 5%
hydrochloric acid (actually, any strong acid will do).
Prepare 50 ml or so of the acidic solution and put
this in a beaker.
![]() Put a stir
bar in the beaker with the acid, and put it on a
stirrer.
Put a stir
bar in the beaker with the acid, and put it on a
stirrer.
![]() Pour the
liquid from the previous experiment in the acid,
while the stirrer is on.
Pour the
liquid from the previous experiment in the acid,
while the stirrer is on.
When
the above steps are performed, then a bright
yellow and pure precipitate of arsenic sulfide is
formed. This precipitate can be filtered and dried
and in this way a very finely powdered purely
yellow pigment can be obtained. In the past, this
process was used to make artificial very pure
yellow pigment, free of brown or reddish
impurities and with very small particle size. The
formation of the
yellow pigment is shown in a video. The
pigment is formed at once.
Dissolving arsenic
sulfide by adding bleach
The
arsenic sulfide in the acidic solution is
remarkably stable. If some of the yellow
precipitate is transferred to concentrated
hydrochloric acid (30% by weight, slightly
fuming), then it still does not dissolve and no
smell of hydrogen sulfide can be observed. This is
in strong contrast to the similar antimony
sulfide, which does dissolve in concentrated
hydrochloric acid and gives a strong smell of
hydrogen sulfide.
When bleach is added to the dilute acid with the
arsenic sulfide, then the solid does dissolve. The
resulting solution is totally clear and pale green
(due to excess bleach and the presence of excess
chlorine, formed in the reaction between the
dilute acid and the bleach). The dissolving of the
arsenic sulfide in acid+bleach is shown in a
video. After two minutes of stirring all arsenic
sulfide is dissolved.
![]()
Discussion of results
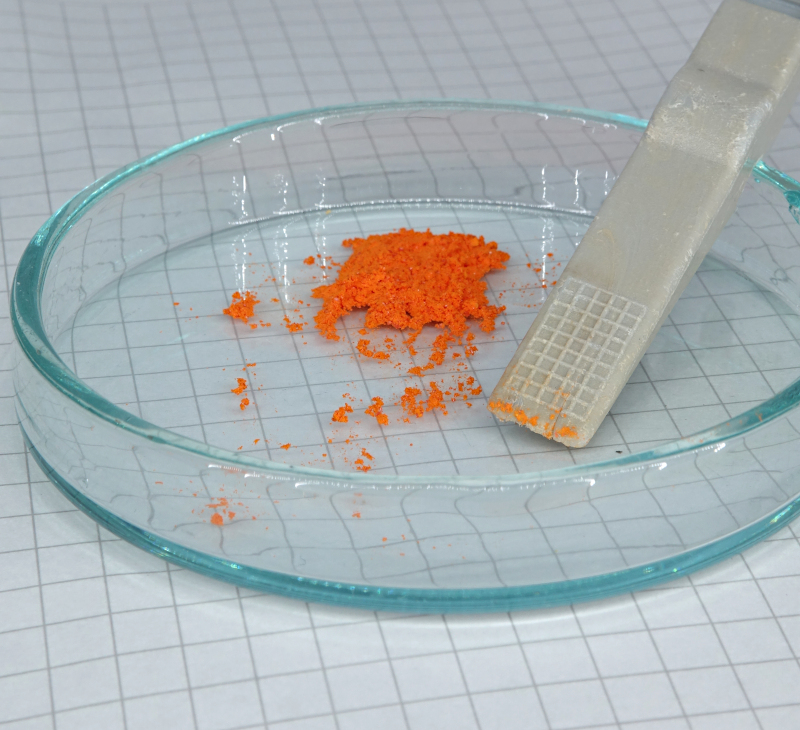
Realgar is a red compound,
which on crunching can be converted to a bright orange
powder. Realgar is not stable to light. Wavelengths in
the range from approximately 500 nm to 670 nm convert
this compound to an allotropic form. In this process,
the crystals crumble to powder with an ochre/orange
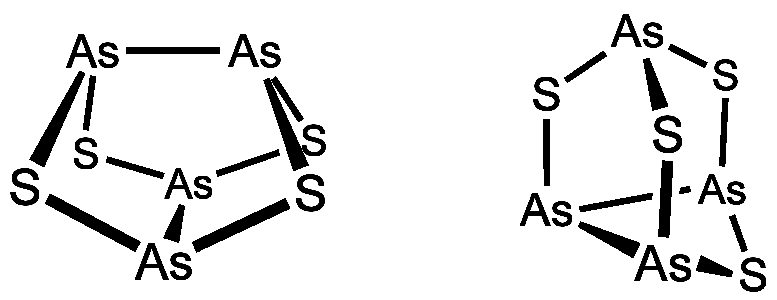
color. The two allotropes of As4S4
have the following structures (left is the red realgar,
right is the ochre/orange pararealgar):
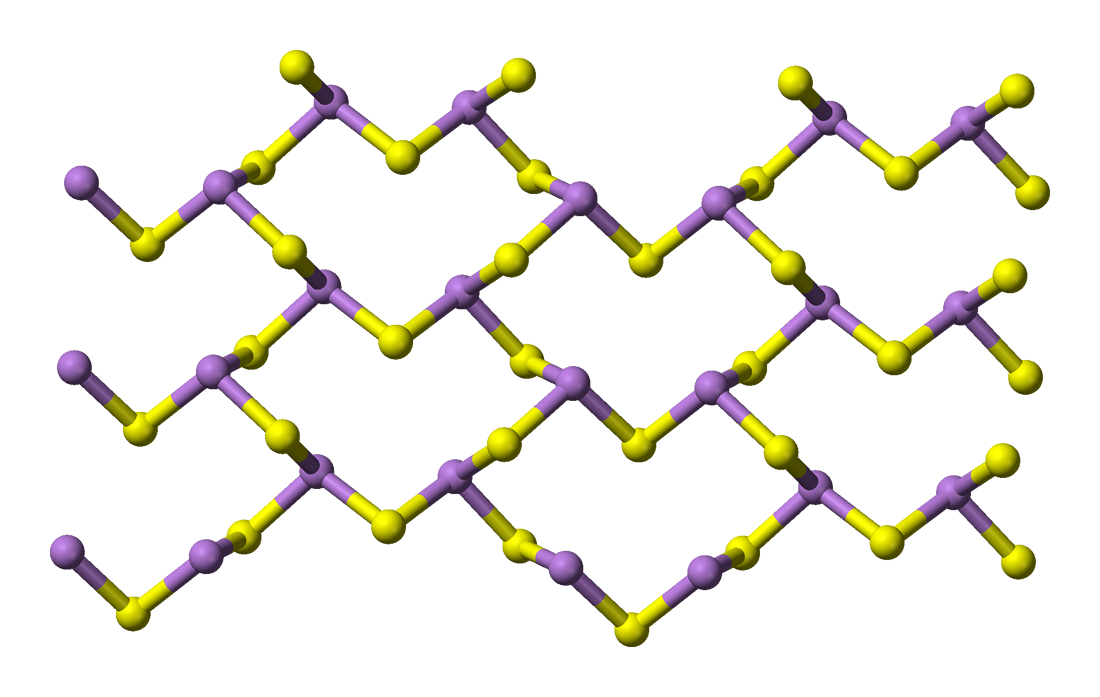
Orpiment is a yellow compound, with a 2D polymeric
structure. It forms sheets of arsenic atoms and sulfur
atoms in a 2 to 3 ratio (see picture below, purple
stands for arsenic, yellow for sulfur).
Solution of realgar in sodium
hydroxide
Realgar dissolves in a solution of
sodium hydroxide. In this compound, arsenic has an
average oxidation state, equal to +2. In the solution,
however, the soluble arsenic species has arsenic in
oxidation state +3. This only is possible if for every
three atoms of arsenic, one is converted to the element
(oxidation state 0), so that the others can go to +3.
The dissolving process goes through complex intermediate
structures with a deep red color of varying composition.
These complex structures are unstable and fall apart in
simple anionic species, which at the very high pH of 10%
solutions of NaOH are derived from orthoarsenite, AsO33–,
with one or two oxygen atoms replaced by sulfur atoms.
So, in solution one gets partial thioarsenites. On
average, the following reaction equation can be given
3 As4S4 + 24 OH–
→ 4 AsOS23–
+ 4 AsO2S3–
+ 12 H2O + 4 As
In reality, the reaction will be more
complicated, and almost certainly, there will also be AsO33–
ions and AsS33–
ions in solution, but on average the ratio of the number
of oxygen atoms and sulfur atoms in the (thio)arsenite
ions will be 1. Maybe a better representation of the
reaction is
3 As4S4 + 24 OH– → 8 "AsO1½ S1½3– " + 12 H2O + 4 As
Here, "AsO1½ S1½3– "
stands for all (partial) thio-orthoarsenite ions, which
on average have the given formula. These (thio)arsenite
ions are colorless. The precipitated arsenic is very
dark brown, nearly black.
Solution of orpiment in sodium hydroxide
Orpiment also dissolves in a solution
of sodium hydroxide. Here, there is no need for the
arsenic to change its oxidation state, and hence no
disproportionation is observed in this reaction. All
arsenic remains in oxidation state +3 and all of it goes
in solution in the form of partial thioarsenites.
As2S3 + 6 OH– → 2 "AsO1½ S1½3– " + 3 H2O
The solution of orpiment in dilute
sodium hydroxide is colorless. If there is some brown
coloration and turbidity, then this can be attributed to
realgar impurities of the orpiment sample. Realgar
impurities are common in samples of orpiment.
Reaction of thioarsenites with
acids
When thioarsenates are acidified, then all sulfide is very tightly bound to arsenic. Arsenic in oxidation state +3 very tightly binds sulfide and even concentrated hydrochloric acid is not capable of releasing the sulfide as H2S from the arsenic. Only if there is excess sulfide, it is released as H2S in acidic solution, but as long as there still is arsenic(III), which is not fully bound to sulfide, then any sulfide present is bound. So, on acidification, some of the following reactions occur:
2 AsS33– + 6 H+ → As2S3 + 3 H2S
2 AsOS23– + 6 H+ → As2S3 + 2 H2O + H2S
AsOS23– + AsO2S3– + 6 H+
→ As2S3 + 3 H2O
H2S in turn reacts with
arsenites and thioarsenites, with the help of some acid,
to form As2S3
and water. An example of such a reaction is the
following:
2 AsO2S3– + H2S + 6 H+
→ As2S3 + 4 H2O
All these reactions finally lead to
formation of As2S3
and binding of all sulfide. These reactions best can be
summarized as follows:
2 "AsO1½
S1½3– " + 6 H+
→ As2S3 + 3 H2O
These reactions all
are driven by the fact that the solubility of As2S3
is extremely low in water and acidic solutions. So, if
any As2S3
is formed, it precipitates and does not take part in
any reaction anymore. This drives the reaction to an
end, until either all arsenic is consumed, or all
sulfide (in the form of H2S in the acidic
solutions) is consumed.
Oxidation of arsenic by bleach and
oxidation of As2S3
by bleach
Arsenic is fairly
easily oxidized. When bleach is present, then the
arsenic is oxidized, all the way to its +5 oxidation
state, and at the high pH of the experimental
conditions, this is in the form of orthoarsenate ion:
2 As + 5 ClO– + 6 OH– → 2 AsO43– + 3 H2O + 5 Cl–
As2S3
is also easily oxidized by bleach. If that reaction
occurs in acidic solution (as was the case in this
experiment), then the arsenate will appear as arsenic
acid. The sulfur is mostly oxidized to sulfate, but
some of it is only oxidized to sulfur. In the acidic
solution, the main reaction can best be represented as
follows:
As2S3 + 14 HClO + 6 H2O →
H3AsO4 + 3 HSO4– + 14 Cl– + 17 H+
In a side
reaction, there also is formation of some
elemental sulfur.