


Ammonium periodate -- a remarkably energetic compound
This is a simple experiment, which is easy to perform, yet it is quite surprising and impressive. In this experiment, some ammonium periodate is made, and this compound is decomposed explosively by heating it.
This is a fairly safe experiment, despite the fact that an explosive compound is made. The only important thing is that this experiment may not be scaled up.
![]()
![]() Required
chemicals:
Required
chemicals:
-
periodic acid
-
ammonia (15% or better, it is important that the ammonia is clean and not full of detergents or other additives.)
-
sodium hydroxide (needed for cleanup)
![]() Required
equipment:
Required
equipment:
- petri dish
- test tube (use an old one, there is a chance of breaking it)
- small burner, used for heating a test tube
![]() Safety:
Safety:
- Ammonia at a concentration of 15% is quite noxious. It has a strong pungent smell. Use adequate ventilation.
- Periodic acid is a strong oxidizer, avoid contact with the skin.
![]() Disposal:
Disposal:
- Let the test tube with the exploded material cool down and then rinse it with a solution of sodium hydroxide, don't use ammonia for cleaning it! The waste may be flushed down the drain.
![]()
Preparation of ammonium periodate
![]() Dissolve a small spatula full of periodic acid in half a ml
of water. Only use a small amount, a few tens of mg.
Dissolve a small spatula full of periodic acid in half a ml
of water. Only use a small amount, a few tens of mg.
![]() Add two ml of 15% clean ammonia.
Add two ml of 15% clean ammonia.
When the ammonia is added, then a compact crystalline precipitate is formed. For convenience, it is best to add the liquids to each other in a petri dish and not in a test tube. The precipitate has a tendency to stick to the glass and this is rather annoying, when this is inside a test tube. Mix the liquids with each other with a small plastic spatula (don't use metal) or a glass rod.
![]() After mixing, spread the liquid with the precipitate over the
petri dish and put the petri dish in a warm place (e.g. on a heat radiator at 50
ēC or so).
After mixing, spread the liquid with the precipitate over the
petri dish and put the petri dish in a warm place (e.g. on a heat radiator at 50
ēC or so).
After one day, the material is dry and has no smell of ammonia anymore.
![]()
Heating of the solid ammonium periodate
Put a small amount of the ammonium periodate in a test tube. In order to get an idea of the small quantity used, have a look at the picture below:

This is a standard test tube (16 cm length with 16 mm diameter) and there only is a thin layer at the bottom. This small amount is heated above the flame of a small alcohol burner. Nothing seems to happen for a while, until suddenly the solid explodes with a loud bang. The following picture shows the test tube, at most 33 ms after the explosion.

A video demonstrates the strong explosion and the formation of the iodine vapor. Download size is approximately 2.6 MByte.
A few minutes after the explosion, the inside walls of the test tube are covered with white material (left over ammonium periodate) and iodine. There also is quite some yellow material. This is due to the presence of iodide and water vapor. This combination, together with iodine, gives the brown triiodide ion, dissolved in water.

![]()
Discussion of results
![]() Periodic acid only exists in the form of orthoperiodic acid, which has the
following structure, according to the formula H5IO6,
better IO(OH)5:
Periodic acid only exists in the form of orthoperiodic acid, which has the
following structure, according to the formula H5IO6,
better IO(OH)5:
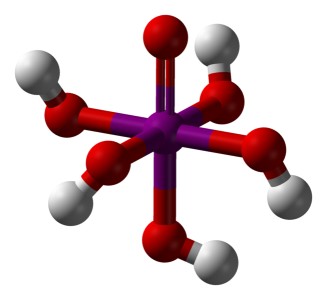
Orthoperiodic acid is a fairly weak acid. When it is dissolved in water, then most of it remains in solution as a molecule. When a weak base is added, then one proton is split off, but further deprotonation requires addition of excess amounts of strong base.
When ammonia is added, then one proton is split off, and an equilibrium occurs between orthoperiodate and metaperiodate:
H5IO6 + NH3 ↔ H4IO6 + NH4+
This equilibrium is shifted to the right to a large extent.
The ion H4IO6 is not very stable and this easily splits off water:
H4IO6 ↔ IO4 + 2H2O
The ion IO4 is called metaperiodate.
The metaperiodate ion is quite remarkable. There are very few cations, which give soluble compounds with metaperiodate (sodium being one of them). Ammonium metaperiodate also is almost insoluble in water. This is what is observed. After addition of the ammonia, a precipitate is formed. This is not formed at once, but it is formed fairly fast, within a few seconds, in the form of a compact fine white crystal mass, which is easily separated from the liquid.
IO4(aq) + NH4+(aq) ↔ NH4IO4(s)
This reaction is almost 100% to the right, even in only moderately concentrated solutions.
Addition of much more ammonia, even strongly concentrated, does not result in redissolving of the white precipitate. Ammonia is not a sufficiently strong base for further deprotonation of orthoperiodate and hence, the metaperiodate ion remains the main periodate species in the system.
Remark: A similar thing happens when a small amount of KOH is added to a solution of periodic acid, such that the first proton of the periodic acid is consumed by the base. In this case, KIO4 is precipitated. If more KOH is added, then the KIO4 redissolves again, and more strongly deprotonated orthoperiodate species are formed, such as H3IO62- and H2IO63-. This latter thing does not happen with ammonia. Ammonia is only a weak base.
![]() Dry ammonium metaperiodate has its own fuel and own
oxidizer in one compound. There is excess oxygen balance, but despite the
non-ideal oxygen balance, this compound is highly energetic. Periodates are very
strong oxidizers and this is perfectly demonstrated by the properties of
ammonium metaperiodate.
Dry ammonium metaperiodate has its own fuel and own
oxidizer in one compound. There is excess oxygen balance, but despite the
non-ideal oxygen balance, this compound is highly energetic. Periodates are very
strong oxidizers and this is perfectly demonstrated by the properties of
ammonium metaperiodate.
When the material explodes, the following main reaction occurs (without doubt there will be numerous side reactions):
2NH4IO4(s) → N2(g) + 4H2O(g) + I2(s, g) + 2O2(g)
As the balanced reaction equation shows, a lot of gases are produced at once, and that makes the explosion particularly powerful.
A test was done with ammonium perchlorate in a similar experiment. This does not explode, but it slowly decomposes, giving water vapor and chlorine gas (faint green color is visible). This also shows that metaperiodate is more energetic than perchlorate. This probably also explains why ammonium metaperiodate is not a chemical of commerce. Ammonium metaperiodate is too dangerous to have around in more than mg quantities.
![]() There also is a side reaction, in which iodide ion is
formed. The presence of the iodide ions explains why there is a yellow/brown
color at the inside of the test tube. Water vapor, produced in the reaction
above, partly dissolves some of the iodide, and then this solution of iodide
reacts with free iodine, giving the brown triiodide ion. The reaction
sequence in which iodide ion is formed most likely is the following:
There also is a side reaction, in which iodide ion is
formed. The presence of the iodide ions explains why there is a yellow/brown
color at the inside of the test tube. Water vapor, produced in the reaction
above, partly dissolves some of the iodide, and then this solution of iodide
reacts with free iodine, giving the brown triiodide ion. The reaction
sequence in which iodide ion is formed most likely is the following:
NH4IO4(s) → NH3(g) + HI(g) + 2O2(g) → NH4I(s) + 2O2(g)
The ammonium iodide dissolves in condensed water from the main reaction, giving iodide ions in the droplets. These iodide ions react with iodine:
I(aq) + I2(s, g) → I3(aq)
This effect of formation of triiodide ion only is visible in the higher parts of the test tube, which were not heated by the flame of the alcohol burner. At those places, the water vapor could condense and dissolve some ammonium iodide. Near the bottom of the test tube, no such yellow material can be observed. At that place, no water is condensed on the glass and so, no triiodide could be formed. Closer to the bottom, solid elemental iodine is deposited on the glass, giving the grey material. At the bottom itself no iodine has settled, that area also was too hot for the iodine to sublime.