


A covalent compound of tin
Tin is a metal and many metals are expected to form ionic salt-like compounds. In this experiment however, the metal is brought to a high oxidation state in combination with iodine and this results in formation of a remarkable and quite interesting covalent compound: tin(IV) iodide, having chemical formula SnI4.
This experiment is also nice because of its simplicity. The covalent compound can easily be isolated in a high state of purity and can be handled without the need of special apparatus. Below, some solid tin(IV) iodide is shown, photographed from within a glass test tube. It is a crystalline bright orange solid.

This webpage gives an outline of how this compound can be prepared and it shows some of the interesting physical properties of this compound. This is not a particularly dangerous experiment as long as the safety guidelines are followed well.
The experiment was tried two times, once with carbon disulfide as solvent and another time with dichloromethane as solvent. Both experiments were successful. When dichloromethane is used, just use a little bit more of the solvent in order to compensate for the somewhat lesser solubility of iodine in dichloromethane, compared to the solubility in carbon disulfide.
![]()
![]() Required
chemicals:
Required
chemicals:
- tin powder
- iodine
- carbon disulfide (or dichloromethane)
![]() Required
equipment:
Required
equipment:
- test tubes
- fairly strong heater (bunsen burner or propane torch)
- safe and stable clamp for holding the test tube
![]() Safety:
Safety:
- Iodine is toxic and corrosive. When crystals of iodine come in contact with the skin, then the skin is stained brown.
- Carbon disulfide is toxic and extremely flammable. Vapor of carbon disulfide can ignite even below 100 °C. One step of the experiment can be dangerous if done carelessly. This step must be done outside or in a place where no vapor can build up and nothing flammable is near by. A good alternative for the use of carbon disulfide is the use of dichloromethane. Dichloromethane is toxic as well (possible carcinogen), but it is not flammable.
- The resulting compound, tin(IV) iodide is corrosive.
![]() Disposal:
Disposal:
- After the experiment, the liquid waste may be flushed down the drain with a lot of water. Only a very small amount of tin is used and the tin goes into the water as hydrous SnO2 which is not particularly toxic.
- Solid remains of tin metal can be wrapped in some paper and thrown in the trash bin.
- In order to clean the test tube, some strong brushing may be necessary. Some of the tin(IV) iodide decomposes on heating and leaves a thin dark stain on the inside of the test tube which only can be removed with strong mechanical action.
![]()
Preparation of SnI4
As a starting material use pure tin metal. Soldering tin is not suitable, this contains too much other metals alloyed with the tin (e.g. lead or silver). The preferred form of the metal is as a powder, but the metal can also be melted and the drops dripped on water or even better on a perfectly dry and clean glass surface, such that the tin has a large surface area for reaction. The tin, used in this experiment was a coarse powder.

The procedure for making tin(IV) iodine is outlined below.
![]() Take a
small amount of tin metal and mix this with iodine. Put the materials in a test
tube. Assure that excess tin metal is used, otherwise the resulting compound
will not be pure, but mixed with hard to remove iodine. The picture below shows
the bottom of an ordinary test tube of 16 mm diameter with a small amount of tin
and iodine mixed with each other. The iodine immediately gives a somewhat brown
color to the tin, but this probably is not due to a reaction, but simply due to
the well-known staining effect of iodine on many materials.
Take a
small amount of tin metal and mix this with iodine. Put the materials in a test
tube. Assure that excess tin metal is used, otherwise the resulting compound
will not be pure, but mixed with hard to remove iodine. The picture below shows
the bottom of an ordinary test tube of 16 mm diameter with a small amount of tin
and iodine mixed with each other. The iodine immediately gives a somewhat brown
color to the tin, but this probably is not due to a reaction, but simply due to
the well-known staining effect of iodine on many materials.

![]() Add
approximately half a ml of carbon disulfide. As soon as that is done, the iodine
dissolves very easily and a deep purple liquid is obtained. Instead of half a ml
of carbon disulfide one can use 1 ml of dichloromethane and follow the procedure
given below.
Add
approximately half a ml of carbon disulfide. As soon as that is done, the iodine
dissolves very easily and a deep purple liquid is obtained. Instead of half a ml
of carbon disulfide one can use 1 ml of dichloromethane and follow the procedure
given below.

Most of the iodine dissolves within a few seconds. The tin remains on the bottom, but in the dark liquid one can hardly see the tin.
![]() Swirl
the test tube for a few minutes. In just a few minutes, the color changes from
deep purple to yellow/orange through shades of red and brown. The liquid
slightly heats up, it feels like a warm hand. The pictures
below show how the liquid changes color. Each picture was made approximately 30
seconds after the previous picture.
Swirl
the test tube for a few minutes. In just a few minutes, the color changes from
deep purple to yellow/orange through shades of red and brown. The liquid
slightly heats up, it feels like a warm hand. The pictures
below show how the liquid changes color. Each picture was made approximately 30
seconds after the previous picture.






Finally, after approximately 5 minutes, a nice clear and orange liquid is obtained.

![]() This liquid is poured over in another test tube, so that no
tin granules are left in the liquid. With this liquid, all further experiments
are done.
This liquid is poured over in another test tube, so that no
tin granules are left in the liquid. With this liquid, all further experiments
are done.

![]() Now, a
solution of tin(IV) iodide in carbon disulfide (or dichloromethane) is obtained. The
solvent
must be boiled away in order to get the pure compound. This can be done easily, because of the low boiling point of the solvent, but when carbon
disulfide is used, one must be
really careful not to get a fire.
Now, a
solution of tin(IV) iodide in carbon disulfide (or dichloromethane) is obtained. The
solvent
must be boiled away in order to get the pure compound. This can be done easily, because of the low boiling point of the solvent, but when carbon
disulfide is used, one must be
really careful not to get a fire.
 Because
this is just half a ml of carbon disulfide it was decided to boil it away using
the flame of a propane torch, which seems very unwise
Because
this is just half a ml of carbon disulfide it was decided to boil it away using
the flame of a propane torch, which seems very unwise
![]() .
This must be done outside on a breezy day.
.
This must be done outside on a breezy day.
If carbon disulfide is used, there is a fairly large risk of the test tube catching fire. If this happens outside, far away from other flammable materials, no real harm is done (it's only 0.5 ml), but if this happens inside, then nasty things can happen, also due to the risk of buildup of high concentrations of vapor around the test tube. If the boiling away of the carbon disulfide is done outside with the test tube in a stable clamp, then the risk is acceptable, but be prepared of a small fire. In case of a small fire (flame coming out of the open end of the test tube) just step back and wait until the fire ends. If dichloromethane is used instead, then there is no risk of fire, but one should assure not to breathe the possibly carcinogenic vapor.
After boiling away the carbon disulfide or dichloromethane and cooling down of the test tube, an orange solid remains behind. At the places where there is a very thin and finely divided layer of solid material, it looks yellow.

A picture of a sample of this material is present on Wikipedia, this picture is reproduced here (with permission of the author).

Remark 1: This experiment can also be done with CH2Cl2 used as solvent instead of CS2. The use of one of CH2Cl2 is safer, because of the lack of risk of fire. Instead of using 0.5 ml, take 1 ml of this solvent. If that is done, then the iodine also dissolves fairly quickly and then the reaction proceeds within a few minutes, just as with carbon disulfide. All pictures made above also could be made with dichloromethane as solvent. The colors are the same, the final solution in dichloromethane is orange, just as in carbon disulfide.
Remark 2: When molten and splashed tin droplets are used instead of tin powder, then the reaction takes a much longer time to proceed. In that case it may take over an hour before all iodine has reacted with the tin, even when carbon disulfide is used.
Remark 3: Always use a fairly large excess of tin. Use of a large excess of tin makes purification of the material very simple because all iodine quickly reacts. The liquid with the dissolved tin(IV) iodide simply can be decanted from excess tin, as described above. When excess iodine is used, then this also remains in solution and the separation of the iodine and tin(IV) iodide is much harder than separation from tin.
![]()
Properties of SnI4
Tin(IV) iodide is a truly covalent compound, which is low-melting and fairly low-boiling. When the test tube with the orange solid is heated carefully, then the solid easily melts, giving a deep red liquid, which in thicker layers almost looks black. The liquid is quite volatile and even a little above the melting point, there also is a clearly visible vapor of tin(IV) iodide. This vapor looks yellow. It condenses on the glass, giving many tiny orange droplets.

When the material is heated more strongly, then the liquid starts boiling vigorously and much darker vapor is produced. The vapor is yellow/brown, somewhat like bromine vapor, but less red, more towards yellow. In cooler parts of the test tube, the vapor condenses on the glass and tin(IV) iodide runs back towards the bottom in thin bright red streams of liquid.

On even stronger heating, the material can be brought to hefty refluxing and dark vapor is produced. The material also seems to decompose somewhat at really high temperatures: the glass is stained somewhat. The vapor now seems to become more red/brown, resembling bromine vapor even more. This shift towards red may be due to slight decomposition, leading to formation of iodine, which has purple vapor and the mix of tin(IV) iodide and traces of iodine may lead to a somewhat more red appearance of the vapor.

After cooling down somewhat, all liquid has collected at the bottom again, the color of the vapor has shifted towards yellow/brown and the liquid is deep red. Still, the test tube is very hot and well above the melting point of tin(IV) iodide.

The liquid in the test tube can be used to wet the inside of the test tube, by carefully turning it around. The wetted test tube shows the beautiful deep red color of the liquid. On cooling down, the material becomes crystalline and the color shifts from red towards orange. The left picture shows the still hot molten material, the right picture shows the same material after cooling down a little below the melting point, but still too hot be be touched with bare hands.


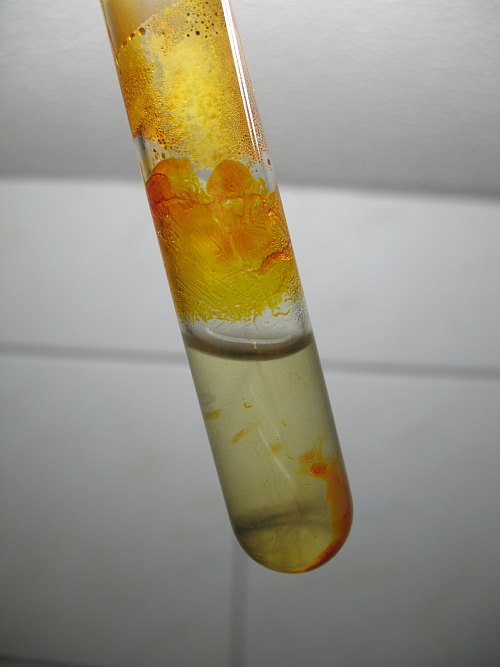
Tin(IV) iodide is hydrolysed by water. This reaction is not violent, but in just a few minutes, it is completely hydrolysed. The picture below shows the completely cooled down test tube with water added carefully, such that only the lower part of the test tube is touched by water. Most of the tin(IV) iodide under the water has been hydrolysed to white/turbid hydrous tin(IV) oxide and hydroiodic acid in just a minute or two. The part which is above the water now is yellow/orange instead of orange/red. So, the color of tin(IV) iodide intensifies on heating.
![]()
Discussion of results
![]() Tin easily reacts with dissolved iodine as follows:
Tin easily reacts with dissolved iodine as follows:
Sn + 2I2 → SnI4
When there is excess of tin, then all iodine quickly reacts, but the tin(IV) iodide does not react with excess tin.
![]() Tin(IV)
iodide is covalent and not a salt. It easily melts and also can easily be
boiled. Its melting point is only 144 °C and its boiling point is 348 °C. But
well below the boiling point, the material already volatilizes easily, giving
rise to clearly visible vapor. The vapor is brown/yellow.
Tin(IV)
iodide is covalent and not a salt. It easily melts and also can easily be
boiled. Its melting point is only 144 °C and its boiling point is 348 °C. But
well below the boiling point, the material already volatilizes easily, giving
rise to clearly visible vapor. The vapor is brown/yellow.
![]() With
water, the material reacts irreversibly, forming hydrous stannic oxide, also
known as "stannic
acid".
With
water, the material reacts irreversibly, forming hydrous stannic oxide, also
known as "stannic
acid".
SnI4 + 3H2O → "H2SnO3" + 4H+ + 4I–
Stannic acid is not a well-defined compound. It better is described as hydrous tin(IV) oxide, hence the use of " " around the formula of stannic acid.
The color of tin(IV) iodide becomes deeper when it becomes hot.