

Formation of a remarkable
volatile molybdenum compound
Molybdenum and its common compounds
all are non-volatile solids. This metal is used in
specialty metallurgical applications, and its compounds
are used in ceramics, as lubricant, and in the
laboratory. In this experiment, a volatile molybdenum
compound is made, which easily can be obtained in the
gaseous state and which can be refluxed in a test tube.
This experiment is quite spectacular, but it must be
carried out carefully.

![]() In this experiment
some phosphorus pentachloride is used, which is
extremely corrosilve and must be handled with great
care. The dark brown fumes, produced in this experiment,
should not be inhaled.
In this experiment
some phosphorus pentachloride is used, which is
extremely corrosilve and must be handled with great
care. The dark brown fumes, produced in this experiment,
should not be inhaled.
![]()
![]() Required chemicals:
Required chemicals:
-
molybdenum trioxide
-
phosphorus pentachloride
![]() Required equipment:
Required equipment:
-
test tube
- small propane torch or alcohol
burner
![]() Safety:
Safety:
- Molybdenum trioxide is fairly toxic. Avoid inhaling dust.
-
 Phosphorus
pentoxide is extremely corrosive and reacts
violently with water. Handle this chemical with
great care and avoid inhaling the fumes, given off
by this compound.
Phosphorus
pentoxide is extremely corrosive and reacts
violently with water. Handle this chemical with
great care and avoid inhaling the fumes, given off
by this compound.
- The dark brown fumes, produced in
this experiment, are corrosive and contain molybdenum.
Avoid inhaling these fumes, they are very toxic.
![]() Disposal:
Disposal:
- The waste of this experiment must be regarded as heavy metal waste. It can best be neutralized somewhat with sodium hydroxide and then it should be brought to a municipal waste processing facility.
![]()
Production of molybdenum
pentachloride
![]() Put some powdered molybdenum
trioxide in a test tube.
Put some powdered molybdenum
trioxide in a test tube.
![]() Add an excess amount of finely
divided solid phosphorus pentachloride to the molybdenum
trioxide and mix both chemicals by carefully shaking the
test tube. After mixing, the molybdenum trioxide turns
somewhat brown already.
Add an excess amount of finely
divided solid phosphorus pentachloride to the molybdenum
trioxide and mix both chemicals by carefully shaking the
test tube. After mixing, the molybdenum trioxide turns
somewhat brown already.

![]() Heat the test tube with a
small flame of a small propane torch or with the flame
of an alcohol burner. Apply the heat just under the mix
of both chemicals.
Heat the test tube with a
small flame of a small propane torch or with the flame
of an alcohol burner. Apply the heat just under the mix
of both chemicals.
When the above instructions
are followed, then after a few seconds a sudden reaction
sets in, in which a lot of brown vapor is produced,
which quickly condenses to a very dark brown liquid,
which runs along the glass.



When the test tube is
gently heated from below, then the brown liquid can be
kept refluxing in the test tube. The liquid, however,
slowly decomposes to a black solid, especially at the
spot, where the test tube is heated. This solid forms an
immobile crust on the glass, which is not volatile.
Formation of the brown vapor
and the refluxing along the glass is shown in this video.
![]() Let
the test tube cool down. This results in most of the
liquid running back to the bottom of the test tube,
where it solidifies to a black solid. The glass remains
covered by a thin brown layer.
Let
the test tube cool down. This results in most of the
liquid running back to the bottom of the test tube,
where it solidifies to a black solid. The glass remains
covered by a thin brown layer.
![]() Add
concentrated hydrochloric acid (appr. 35% HCl by weight)
to the test tube. The solid dissolves in the
hydrochloric acid and it forms a beautiful bright green
solution. The non-volatile black compound does not
dissolve in the acid (or it dissolves very slowly).
Add
concentrated hydrochloric acid (appr. 35% HCl by weight)
to the test tube. The solid dissolves in the
hydrochloric acid and it forms a beautiful bright green
solution. The non-volatile black compound does not
dissolve in the acid (or it dissolves very slowly).

This video
shows how the volatile compound dissolves in the acid.
![]() Dilute
the acid with a lot of distilled water. When this is
done, then the green compound changes to a brown
compound. The solid black particles do not change.
Dilute
the acid with a lot of distilled water. When this is
done, then the green compound changes to a brown
compound. The solid black particles do not change.

![]()
Discussion of results
The reactions, shown in this
experiment, are quite uncommon. Only few chemists know
this kind of chemistry of the element molybdenum.
![]() Phosphorus pentachloride is a
very strongly chlorinating reactant. It is capable of
replacing oxygen by chlorine atoms in a wide range of
compounds. In molybdenum trioxide it is capable of
replacing each of the three oxygen atoms by two chlorine
atoms.
Phosphorus pentachloride is a
very strongly chlorinating reactant. It is capable of
replacing oxygen by chlorine atoms in a wide range of
compounds. In molybdenum trioxide it is capable of
replacing each of the three oxygen atoms by two chlorine
atoms.
3
PCl5 + MoO3 →
3 POCl3 +
MoCl6
The compound MoCl6 is not
stable, not even at room temperature, and certainly not
at the elevated temperatures of the heated test tube.
The compound MoCl6
quickly decomposes by splitting off a chlorine atom:
2 MoCl6 → 2 MoCl5 + Cl2
The compound MoCl5 is a
volatile compound, which easily forms dark red/brown
vapors and a very dark brown liquid. The solid is black.
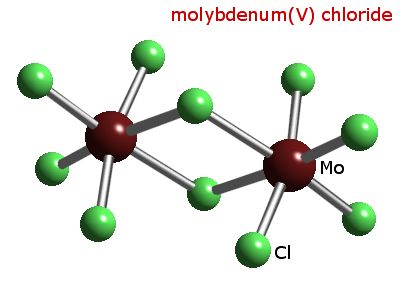
In the solid state it exists as its dimer Mo2Cl10,
which has two bridging chlorine atoms between the two
molybdenum atoms. In the gas phase, it exists as
monomer.
On stronger heating, the pentachloride
loses an additional chlorine atom, giving molybdenum(IV)
chloride. This in turn easily loses another chlorine
atom, giving molybdenum(III) chloride. Molybdenum(IV)
chloride is a black compound, which slowly dissolves in
water, giving black molybdenum(IV) oxo-compounds and
hydrogen chloride. Molybdenum(III) chloride is a very
dark red compound, insoluble in water.
MoCl5 + H2O → MoOCl3 + 2 HCl
In highly concentrated hydrochloric acid it forms a complex MoOCl3 · 2 HCl. Probably, in strongly acidic aqueous solution it exists as MoOCl52–. This complex has a bright green color.
On dilution, there is further hydrolysis, which results in formation of a brown compound. The composition of that brown compound is not known to me, the author of this webpage. It most likely will have more oxygen atoms attached to the molybdenum atom and more chloride will have hydrolysed to HCl.