


Isolation of a peroxo complex of vanadium
Chromium is known to form many peroxo complexes, some of them having beautiful colors and all of them being rather energetic. It is much less well-known that vanadium in its +5 oxidation state also can form nice peroxo complexes, which also are colorful and energetic. The two pictures below show the violent explosion of a tiny amount of the mix of red phosphorus and the vanadium peroxo complex. Time between the two frames is 33 ms.


 This
experiment can be conducted safely, but one should not scale up the explosions,
as shown in the experiment. The peroxo complex, formed in this experiment is
fairly stable and is not as dangerous as the peroxo complexes, formed with
hexavalent chromium, but still, there always is the risk of unexpected and
premature ignition of a mix of the peroxo complex and phosphorus, so never
prepare more than a few tens of mg of this mix and do the mixing very carefully.
Also assure that the smoke, produced in this reaction, is not breathed. This
experiment must be performed in a well ventilated room, or even better, outside
or in a fume hood.
This
experiment can be conducted safely, but one should not scale up the explosions,
as shown in the experiment. The peroxo complex, formed in this experiment is
fairly stable and is not as dangerous as the peroxo complexes, formed with
hexavalent chromium, but still, there always is the risk of unexpected and
premature ignition of a mix of the peroxo complex and phosphorus, so never
prepare more than a few tens of mg of this mix and do the mixing very carefully.
Also assure that the smoke, produced in this reaction, is not breathed. This
experiment must be performed in a well ventilated room, or even better, outside
or in a fume hood.
In this webpage, two experiments are described. The first part of the experiment can easily be performed, and only requires aqueous chemistry without the need to isolate any compounds. It already is quite interesting on its own. The second part of the experiment requires filtration and drying of a solid and requires a little bit more experience.
![]()
![]() Required
chemicals:
Required
chemicals:
-
vanadium pentoxide
-
potassium hydroxide
-
hydrogen peroxide (15%)
-
ethanol
-
dilute sulphuric acid
-
red phosphorus
![]() Required
equipment:
Required
equipment:
-
test tubes
-
a small beaker
-
funnel and filter paper
-
small alcohol burner and metal spatula (screw driver also works)
![]() Safety:
Safety:
- Vanadium pentoxide and the vanadium compounds, formed in this experiment are toxic. Avoid breathing any of the dusts and the smoke.
- Potassium hydroxide is very corrosive. Never allow this compound to get into the eye, it will cause quick and permanent damage! Use good goggles. If this comes in contact with the skin, then rinse with water, until the slippery/soapy feeling has gone.
- Ethanol is flammable.
- Dilute sulphuric acid is corrosive, avoid contact with skin and eyes.
- Red phosphorus is flammable.
![]() Disposal:
Disposal:
- The waste of vanadium salts should not be flushed down the drain. Keep this waste and bring it to a proper waste processing facility as heavy metal waste.
![]()
Formation of a peroxo complex in aqueous solution
![]() Take
some powdered vanadium pentoxide and add some water to this. When this is done,
then it hardly dissolves, it is dispersed in the water, but most of it settles
at the bottom.
Take
some powdered vanadium pentoxide and add some water to this. When this is done,
then it hardly dissolves, it is dispersed in the water, but most of it settles
at the bottom.


![]() Add some
solid potassium hydroxide to the test tube with the brown/yellow solid and the
yellow material, which is finely dispersed in the water. When sufficient
potassium hydroxide is added, then all of the yellow solid dissolves and a pale
yellow solution is obtained. The two pictures below show the result of adding
sufficient potassium hydroxide. First, the liquid remains somewhat turbid and
light yellow, but after a few minutes, it is almost colorless. A faint yellow
color remains and sometimes some turbidity remains as well. This probably is due
to some impurity in the vanadium pentoxide. The liquid, shown in the pictures
below is strongly alkaline and contains a large excess of potassium hydroxide.
Add some
solid potassium hydroxide to the test tube with the brown/yellow solid and the
yellow material, which is finely dispersed in the water. When sufficient
potassium hydroxide is added, then all of the yellow solid dissolves and a pale
yellow solution is obtained. The two pictures below show the result of adding
sufficient potassium hydroxide. First, the liquid remains somewhat turbid and
light yellow, but after a few minutes, it is almost colorless. A faint yellow
color remains and sometimes some turbidity remains as well. This probably is due
to some impurity in the vanadium pentoxide. The liquid, shown in the pictures
below is strongly alkaline and contains a large excess of potassium hydroxide.


![]() Let any solid matter settle at the bottom or use a very fine
filter to get a clear and almost colorless solution. To this colorless solution
add some 15% hydrogen peroxide (if you have a stronger solution, first dilute
this). When the 15% hydrogen peroxide is added, then the liquid turns deep
yellow at once. When there is a large excess amount of hydrogen peroxide, then
the liquid turns purple with a blue hue. If only a small amount of hydrogen
peroxide is added, then the liquid remains yellow.
Let any solid matter settle at the bottom or use a very fine
filter to get a clear and almost colorless solution. To this colorless solution
add some 15% hydrogen peroxide (if you have a stronger solution, first dilute
this). When the 15% hydrogen peroxide is added, then the liquid turns deep
yellow at once. When there is a large excess amount of hydrogen peroxide, then
the liquid turns purple with a blue hue. If only a small amount of hydrogen
peroxide is added, then the liquid remains yellow.
The picture below shows the result of adding a solution of 15% hydrogen peroxide to the liquid, shown above. The solution of hydrogen peroxide has a lower density than the alkaline solution with dissolved vanadium pentoxide. For this reason, the concentration of hydrogen peroxide remains fairly low in the lower parts of the test tube, while it is highest in the higher part. The lower part remains yellow, the higher part shows the formation of a purple compound. There also is slow formation of bubbles of oxygen.

A video is made of the formation of the complex and the mixing of the liquid. Download size is 3.3 MByte.
![]() When more hydrogen peroxide is added and the liquid is mixed
well, then the liquid turns very dark, almost black. The real color of this
liquid is deep purple. The purple color can be observed when the light of a lamp
is viewed through the liquid (an example is shown further below in this
webpage).
When more hydrogen peroxide is added and the liquid is mixed
well, then the liquid turns very dark, almost black. The real color of this
liquid is deep purple. The purple color can be observed when the light of a lamp
is viewed through the liquid (an example is shown further below in this
webpage).
![]()
Isolation of a peroxo complex
It is fairly easy to isolate a peroxo complex from the solutions, as shown above. One should prepare the dark purple solution, according to the procedure, shown above. The light yellow solution, from which one starts, does not need to be highly concentrated. Take a big spatula full of vanadium pentoxide, add a few ml of water and then add potassium hydroxide, until all of the vanadium pentoxide has dissolved. Then add a few more pieces of potassium hydroxide and dissolve these as well. In this way, a strongly alkaline solution is obtained. Let this solution cool down to 10 °C.
![]() To this solution, a large excess of 15% hydrogen peroxide
must be added. The hydrogen peroxide also must be cold, 5 °C or so.
To this solution, a large excess of 15% hydrogen peroxide
must be added. The hydrogen peroxide also must be cold, 5 °C or so.
![]() When the solution has turned very dark, then add the same
volume of clean ethanol (denatured is suitable, but it should not be loaded with
dyes, perfumes or cleaning stuff). When the ethanol is added, then a light
brown/yellow precipitate is formed. This precipitate is hard to see in the very
dark liquid.
When the solution has turned very dark, then add the same
volume of clean ethanol (denatured is suitable, but it should not be loaded with
dyes, perfumes or cleaning stuff). When the ethanol is added, then a light
brown/yellow precipitate is formed. This precipitate is hard to see in the very
dark liquid.
![]() Pour the solution, with the light brown/yellow solid in it,
in a filter and let gravity do its work. This may take quite a long time.
Pour the solution, with the light brown/yellow solid in it,
in a filter and let gravity do its work. This may take quite a long time.
After a single pass of filtering, a dirty light brown slurry is obtained, and a deep purple clear liquid.

This picture shows the dark color of the liquid after filtering. It is almost black.

But, when the light of a fluorescent tube is viewed through the liquid, then one can nicely see the purple color of this liquid. The ethanol apparently does not react with the peroxo complex, it is not destroyed by the ethanol. The black spots in the purple band are due to bubbles of oxygen in the liquid.

![]() When the liquid is diluted with water, then the purple color
quickly fades and the color shifts towards grey. The picture below shows the
result of diluting the liquid approximately 10 times with water. When the liquid
is allowed to stand for a longer time, then the color further shifts to grey
with a yellow/brown tinge.
When the liquid is diluted with water, then the purple color
quickly fades and the color shifts towards grey. The picture below shows the
result of diluting the liquid approximately 10 times with water. When the liquid
is allowed to stand for a longer time, then the color further shifts to grey
with a yellow/brown tinge.

![]() When the
grey dilute liquid is acidified with excess dilute sulphuric acid, then the
color shifts from grey to deep red/brown. The red/brown color is really intense.
The picture, shown below, shows a solution which already is very dilute in
vanadium content.
When the
grey dilute liquid is acidified with excess dilute sulphuric acid, then the
color shifts from grey to deep red/brown. The red/brown color is really intense.
The picture, shown below, shows a solution which already is very dilute in
vanadium content.

![]() The
dirty light brown slurry is not yet allowed to dry. It is rinsed with cold
distilled water one time. When the water is added, then the liquid becomes
fairly dark, but after filtering a much cleaner-looking precipitate remains.
The
dirty light brown slurry is not yet allowed to dry. It is rinsed with cold
distilled water one time. When the water is added, then the liquid becomes
fairly dark, but after filtering a much cleaner-looking precipitate remains.
The picture below shows the result of adding some distilled water, which rinses the precipitate. The precipitate hardly dissolves in the cold water.

When all water has gone through the filter, then a yellow/brown precipitate of a more bright color remains.

This precipitate is carefully scraped off the filter paper and put in a petri dish, which then is placed in a warm place (on a heating radiator of appr. 50 C). After a few hours, the solid has dried and can be scraped from the glass of the petri dish. The result is a brown/yellow solid, which is not hygroscopic. The solid does not look crystalline, it is more like a fine powder.
The picture below shows the isolated yellow/brown compound and for comparison, a commercial sample of potassium metavanadate is put besides it. The peroxo complex clearly has a darker color.

![]() The dark purple liquid, which remained after filtering, is
not stable. When it is allowed to stand for a day, a yellow precipitate is
formed, which settles at the bottom and the glass wall of the test tube. The
pictures below show the liquid, immediately after filtration, and one day later.
The dark purple liquid, which remained after filtering, is
not stable. When it is allowed to stand for a day, a yellow precipitate is
formed, which settles at the bottom and the glass wall of the test tube. The
pictures below show the liquid, immediately after filtration, and one day later.


![]()
Energetic properties of the isolated peroxo complex
The peroxo complex is expected to be an energetic compound, due to the high oxygen content, which also is present in the form of the relatively unstable peroxo-ligand. For this purpose, some of the dry brown/yellow powder was carefully mixed with a small amount of red phosphorus. No precise weighing was done, the mix was prepared visually, taking approximately 2 times as much volume of the light brown/yellow powder, as volume of powder of red phosphorus. Only very small amounts were mixed.
The mix then was put on the tip of a small screw driver (diameter of tip is 2.5 mm) and kept above a flame. This results in a remarkably violent deflagration. The mix explodes in a very particular way, spraying around smoking sparks in the form of a hairy sphere. Below, 5 frames are shown, which in total span a time of approximately 150 ms.
The first picture shows how small an amount of solid is used. The second picture shows the violent explosion, in which glowing particles are blown away with force (the purple haze is due to a camera lens flare artifact). The large picture shows the afterglow, the explosion itself already is over in this situation. The last two pictures show the nice pattern of smoke, which remains after the explosion.
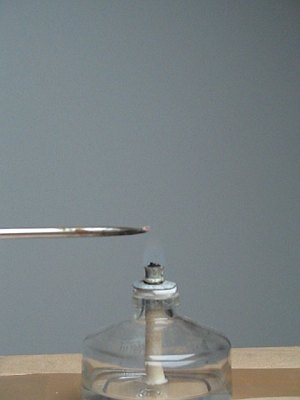




The pictures at the start of this webpage show the explosion of a somewhat larger amount (approximately 2 times the amount, shown here). A video of a few explosions demonstrates the noise and the intensity of the reaction. Download size of the video is 2.1 MByte.
![]()
Stability of the isolated peroxo complex
The solid compound was stored for 50 days in its small glass vial at a temperature of approximately 15 °C. After that period the mix with red phosphorus was tested again for its energetic properties. The compound still is as energetic as the freshly prepared compound. Apparently the material can be kept around for a longer time and does not quickly decompose.
![]()
Discussion of results
![]() Vanadium pentoxide easily dissolves in strongly alkaline solutions. At very high
pH, this leads to formation of the colorless orthovanadates, and somewhat
protonated species. A very strong simplification is the following reaction
equation:
Vanadium pentoxide easily dissolves in strongly alkaline solutions. At very high
pH, this leads to formation of the colorless orthovanadates, and somewhat
protonated species. A very strong simplification is the following reaction
equation:
V2O5 + 6OH– → 2VO43- + 3H2O
In reality, the behavior of vanadium in oxidation state +5 is amazingly complex and not a single simple species is formed, but a highly complicated mix of all kinds of ions. Equilibria like the following occur in the solution:
VO43- + 2H2O ↔ VO2(OH)2– + 2OH–
2VO43- + H2O ↔ V2O74- + 2OH–
![]() When hydrogen peroxide is added to this strongly
alkaline solution, then a yellow complex is formed. A simplified reaction
equation is the following:
When hydrogen peroxide is added to this strongly
alkaline solution, then a yellow complex is formed. A simplified reaction
equation is the following:
VO43- + 2H2O2 → VO2(O2)23- + 2H2O
Probably a more complicated reaction occurs, in which hydroxo-ligands are involved as well, based on the equilibria of the vanadium(V) species.
VO2(OH)2– + 2H2O2 → V(O2)2(OH)2– + 2H2O
The yellow solution contains a mix of VO2(O2)23- and protonated derived species.
![]() At a large excess amount of hydrogen peroxide, ligand exchange even goes
further. Oxo ligands and hydroxo ligands are replaced by peroxo ligands. For
replacement of oxo ligands this looks as follows:
At a large excess amount of hydrogen peroxide, ligand exchange even goes
further. Oxo ligands and hydroxo ligands are replaced by peroxo ligands. For
replacement of oxo ligands this looks as follows:
VO2(O2)23- + 2H2O2 ↔ V(O2)43- + 2H2O
This complex ion V(O2)43- has a deep blue color. When this is the main species, but there also still is some VO2(O2)23-, then the solution may almost look black (absorption of light in the blue tail of the spectrum and in the yellow tail of the spectrum) and the color shifts from blue towards purple or dark grey. This also explains the grey color of the diluted solution. In this solution, the equilibrium slowly goes to the left again and the mix of blue compound and yellow compound results in a grey liquid.
![]() Acidification with a large excess of acid results in formation of a cationic
peroxo-species, which has a dark brown/red color.
Oxo-ligands, hydroxo-ligands and peroxo-ligands all are easily protonated and
this process finally leads to formation of a cationic species. The following
reaction equation describes this process for protonation of the VO2(O2)23-
ion:
Acidification with a large excess of acid results in formation of a cationic
peroxo-species, which has a dark brown/red color.
Oxo-ligands, hydroxo-ligands and peroxo-ligands all are easily protonated and
this process finally leads to formation of a cationic species. The following
reaction equation describes this process for protonation of the VO2(O2)23-
ion:
VO2(O2)23- + 4H+ → VO(O2)+ + H2O + H2O2
This equation is simplified again. In reality, the cationic species also will have a (varying) number of aqua ligands.
![]() Obtaining crystals of the blue species is very difficult and requires salting
out of solutions which are highly concentrated in hydrogen peroxide. This is not
without risk. The yellow species, however, can be separated more easily. Adding
ethanol to a moderately concentrated solution leads to formation of a solid
compound with composition K[V(O2)2(OH)2]∙H2O.
This latter compound frequently is written as KVO4∙2H2O or
even as KVO4.
Obtaining crystals of the blue species is very difficult and requires salting
out of solutions which are highly concentrated in hydrogen peroxide. This is not
without risk. The yellow species, however, can be separated more easily. Adding
ethanol to a moderately concentrated solution leads to formation of a solid
compound with composition K[V(O2)2(OH)2]∙H2O.
This latter compound frequently is written as KVO4∙2H2O or
even as KVO4.
Information about the peroxo-species is from the book Lehrbuch der Anorganischen Chemie, Band II by Heinrich Remy, 1954.